What Makes Metals So Marvelous?

The 2019 National Chemistry Week (October 20-26) theme is “Marvelous Metals,” and no wonder. These ubiquitous elements, which make up more than 75% of the periodic table, are everywhere doing amazing things. The cobalt in vitamin B12 helps us make red blood cells, the iron in hemoglobin carries oxygen to tissues, and sodium and potassium ions allow the heart and nerves to communicate.
Want to get from city to city or around the world without metals—forget it. Aluminum makes aviation possible, and steel (mostly iron) forms the frames of cars, buses, and trains. Not to mention the copper, silicon, and rare-earth metals that make all of our electrical power, computers, and smart devices possible.
And the possibilities keep growing. From gold nanoparticles that can detect and treat cancer to vanadium-stabilized batteries to aluminum-based materials that can harvest water from desert air, metals are humanity’s past, present, and future.
You already know some technical basics about metals. They are shiny, malleable, fusible, ductile, good conductors of electricity and heat, and inclined to give up electrons to form cations. But do you know why? What makes metals so marvelous? The answer, of course, lies at the atomic scale.
Getting into shape
Metals are malleable, meaning that they can be formed into other shapes, such as thin sheets or foils, without breaking or cracking. They are also ductile, which means they can be easily drawn into wires. While aluminum foil in your kitchen and copper electrical wires in electric cables are obvious everyday uses of these properties, wires can be shrunk down in complex ways. They can be formed into microcircuits for cell phones, or used in the development of smart coatings for glass that not only make windows reflective but also protective by blocking infrared radiation.
How is it possible for metals to flex in so many different ways? The answer is metallic bonding. In chemistry class, you probably focused on molecular and ionic bonds, in which electrons are shared to varying degrees between individual atoms. In ionic and covalent materials, solids result from strong ionic bonds or the intermolecular forces between molecules. If stress is placed on these substances (i.e., if you bend or stretch them), the bonds/forces holding them together break, and the structure snaps.
In metallic bonds, electrons move freely among the atomic nuclei—they are delocalized, so there isn’t a definitive bond in the way molecular and ionic bonds form. When stress is applied, the electrons simply slip over to an adjacent nucleus. Metallic bonding—and the loosely held electrons—is also why metals conduct electricity so well. Electricity is the flow of electrons. And since metals don’t tightly hold on to electrons during metallic bonding, they are easily replaced.
It can be easy to underestimate the importance of these metallic bonds. But the Statue of Liberty is a 46-meter (151 ft.) iron frame covered with a layer of copper that is 2.3 mm thick. The torch has an additional layer of gold leaf, which is gold beaten into sheets about 0.1–0.2 mm thick. These metals were chosen for their respective qualities: iron’s strength (the statue weighs more than 150 tons), copper’s flexibility (by design, the statue sways several inches in high winds), and gold’s beauty and durability.
To see why metals are ductile, you need to go one step further—to the crystal structure of metals. Because of metals’ malleability, you may not think of metals as crystalline. But metals do organize themselves in unit cell structures. Unit cells that allow planes of atoms to slip past each other in more directions, such as face-centered cubic (i.e., copper, silver, or gold), are far more ductile than those with hexagonal close-packed structure (i.e., cobalt or titanium). Body-centered cubic structures fall in between.
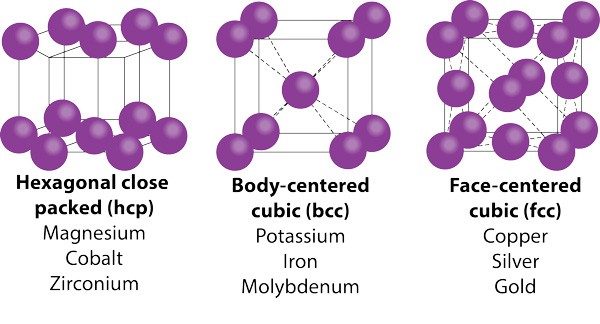
Note that the three most ductile elements on the periodic table—copper, silver, and gold—are all face-centered cubic. One ounce of gold can be drawn into a wire 80-km long and can even be used as thread for embroidery. For smaller wires (think of the wires in your phone’s microcircuitry), metals may be dispersed in a solvent and deposited better size control.
All that glitters
Metals like copper, platinum, silver, and gold are shiny and glitter when polished. This characteristic has a name: luster. Luster is a mineralogical term used to describe the way that light interacts with the surface. It also implies radiance, gloss, or brilliance. You may have noticed that transition-metal-containing compounds (in which the metal is ionized and thus behaves like an ionic solid) are brightly colored; bright-blue CuSO4 , deep-purple KMnO4, or vivid-green Cr2O3. Color in the transition metals results from electronic transitions between the d-level orbitals. Light hitting the compound causes an excitation in the electrons, which results in the electrons jumping to higher energy states. As the electrons relax, the photons that are released have wavelength within the visible portion of the electromagnetic spectrum. For example, compounds made with vanadium can be purplish (+2), greenish (+3), bluish (+4), and yellowish (+5) depending upon the particular oxidation state of vanadium. (Vanadium’s name comes from “Vanadis,” one of the names for the Norse goddess of beauty, after the element’s brightly colored compounds.)
In non-ionized metals, molecular orbitals are in contiunuous movement on the metal surface. This fluidity of metal electrons means that the molecular orbitals have variable, small gaps constantly spitting light back out across a range of wavelengths, giving them a white luster. Copper looks red because it happens to preferentially absorb light in the blue end of the spectrum and emit it in the red. Additional relativistic effects make gold appear yellow.
Silver is the most reflective element. A thin layer on glass (combined with a protective coating to prevent tarnish) is used to make all types of mirrors, from inexpensive hand mirrors to the high-precision mirrors of space telescopes.
Likewise, magnetism results from the motion of the electrons in the material. The metals iron, cobalt, and nickel (all magnetic) have unpaired electrons that can be oriented so that they spin in alignment with one another forming a dipole, which produces the magnetic behavior. Rare-earth metals have even more magnetic behavior, as they can have up to 14 f-orbital electrons (as opposed to transition metals’ 10 d-orbital electrons). Some even expand in the presence of a magnetic field, forming the basis for electronic speakers and buzzers.
Metals react
Just because metals are really flexible doesn’t mean they aren’t reactive. Elements on the left-hand side of the periodic table prefer to give up their electrons, to form ionic compounds. Pure lithium, sodium, potassium, and other Group 1 metals are so reactive they are stored away from air or water. But their ions make up a large portion of the electrolytes our nerves need to transmit electrical signals.
Aluminum is also reactive, albeit less violently than Group 1 elements. But it oxidizes so quickly that it was unknown as an element from around 500 BCE until the early 1800s, even though it is the most abundant metallic element in Earth’s crust. Once the lightweight, sturdy, easy-to-alloy metal was uncovered, it was valued more than platinum, literally! Napoleon III served state dinners featuring aluminum plates and cutlery. It was also used as jewelry and capped the Washington Monument. The 1886 development of an electrolysis-based procedure by American chemist and inventor Charles Martin Hall and French scientist Paul Heroult (working independently) finally made aluminum easy to obtain thereby devaluing it.
No doubt that metals are marvelous. Find out more at the National Chemistry Week website.