Modern Alchemy: Creating Superheavy Elements

Medieval alchemists dreamed of turning lead into gold, of transmuting one element into another. In modern nuclear chemistry, scientists do this all the time—throw atoms together to create new ones that don’t exist outside of a laboratory. These new elements, which reside at the very bottom of the main section of the periodic table, are called the superheavy elements (SHEs) because of their large atomic numbers. The term superheavy is not strictly defined, but it generally includes elements 104 and beyond. You won’t find these elements anywhere within Earth’s crust, and you probably won’t find them in the rest of the universe, but scientists have found ways to synthesize them.
Most methods for making new elements involve a cyclotron, which speeds up atoms to high velocities before they smash into other atoms—these atoms are usually of different elements. This causes the nuclei to combine, creating new heavier elements. Although this process is too expensive and inefficient to mass-produce gold, it is useful for studying the nature of the universe and the limits of the periodic table.
Dawn Shaughnessy leads the team at Lawrence Livermore National Laboratory (LLNL) in Livermore, CA, and collaborates with the Russian Joint Institute for Nuclear Research (JINR) to search for SHEs. This collaboration has resulted in the creation of five new elements (114, 115, 116, 117, and 118) and fundamental changes in the way we view the nuclear atomic structure.
How do we make new elements? Dawn Shaughnessy of Lawrence Livermore National Laboratory explains cyclotrons are used to create new elements.
Models of Nuclei
Because of Ernest Rutherford’s discovery of the nucleus of the atom in 1911, scientists have been able to develop the liquid-drop model to describe its contents. In this model, nucleons (i.e., protons and neutrons) behave like an incompressible liquid. The particles swarm together through a complex interplay of attracting and repelling forces; as more nucleons are added to the mix, the repelling forces become stronger than the attracting ones, and the nucleus becomes unstable.
According to this model, the periodic table should end around element 100 because elements that large should immediately fall apart as a result of instability. But in reality, some large elements, such as dubnium (atomic number 105), stick around for hours.
“Nucleons tend to pair up and populate shells analogous to the way electrons fill their shells,” explains Shaughnessy. “Much like closed electronic shells, each nuclear shell is populated by a certain number of either protons or neutrons. A filled shell represents a more stable nuclear configuration that is resistant to decay.”
Mallory Smith, a beam physicist at the National Superconducting Cyclotron Laboratory at Michigan State University, says that there are multiple forces operating in the quarks that compose nucleons. “The quarks have the fundamental property of having the strong force, one of the four fundamental forces of nature,” Smith explains. This strong force causes quarks to combine into protons and neutrons, but after the quarks combine to form nucleons, there’s strong force left over. “As a result, they [the nuclei] have a strong, attractive, short-range force, and that force is what makes nuclei stick together,” Smith explains.
Protons aren’t exactly happy snuggling close to other protons though. Smith says being positively charged, protons “feel Coulomb repulsion,” which is the repulsion between two like-charged particles. Neutrally charged neutrons don’t feel Coulomb repulsion, so they help stabilize the nucleus: “Because protons want to repel, you need neutrons to make the nucleus stay together,” Smith explains. “And that’s why you can’t have an arbitrary number of protons and neutrons. Because as you get more and more protons, you need more nuclear [strong] force to overcome the Coulomb repulsion. So you need more neutrons for heavier elements.”
Although the liquid-drop model describes this balance between the strong force and Coulomb repulsion for smaller nuclei, it cannot explain why certain numbers of nucleons form surprisingly stable nuclei of heavier isotopes. These “magic numbers” led scientists to develop the shell model. As the name suggests, this model suggests protons and neutrons arrange themselves in shells (similar to electron organization), with filled shells being particularly stable.
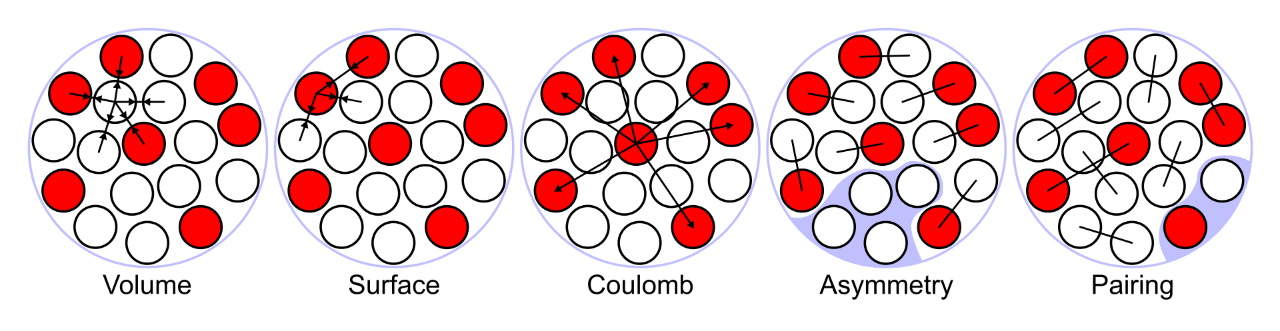
Representation of the energies at play in the liquid drop model of the atomic nucleus.
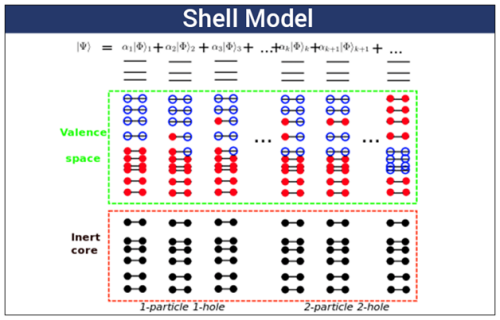
The nuclear shell model explains the distribution of energy level into different atom shells and nucleus atom shells.

Islands of Stability
Magic numbers result in isotopes often referred to as islands of stability within an isotopic sea of instability. In 1998, such an island was almost achieved when scientists from LLNL, including Shaughnessy, and JINR discovered element 114, flerovium. Flerovium is relatively stable for such a large element, lasting for 30 seconds whereas the elements on either side of it exist for mere microseconds.
The isotopes of flerovium that have been made only have filled proton shells, not filled neutron shells. So there could be a more stable isotope that hasn’t been isolated yet; having both shells filled would make the element doubly magic and incredibly stable. As of now, 208Pb is the heaviest doubly magic element: It has a filled proton shell with 82 protons and a filled neutron shell with 126 neutrons.
Even though scientists have been working to approach the superheavy island of stability since the 1960s, the limits of technology currently prevent scientists from creating a doubly magic Fl isotope, which would have 114 protons and 126 neutrons. If the stability of such an isotope could be verified empirically, it would confirm 114 and 126 as magic numbers and add weight to the shell model of the nucleus. “If it is not, then it shows that more work needs to be done on the theory,” says Shaughnessy.
Luckily, scientists are actively developing the technology and investigating ways to make heavier and heavier elements. As a graduate student, Charles “Cody” M. Folden III, associate professor of chemistry at Texas A&M University, played a role in analyzing data used to confirm the discovery of element 111, roentgenium. He now leads a research group that studies why certain nuclear reactions lead to SHEs while others do not. “In the last several years, several elements were discovered using reactions of a neutron-rich nucleus, 48Ca, fusing with targets of actinide elements,” Folden says. Generation of new elements from that type of reaction seems to have plateaued, so Folden is trying to identify new reactions.
Chemical Properties
Beyond nuclear chemistry, superheavy elements can answer questions about the structure of the periodic table itself. Some scientists are also studying the chemical properties of SHEs to determine whether they really resemble other elements in their groups. (For example, does tennessine behave like the other halogens or more like an actinide?)
When nuclei get large, their properties can deviate from the properties of other, smaller elements in the periodic table. According to Folden, whose team is also developing techniques to investigate this strange superheavy chemistry, these properties relate to the influence of relativistic effects. “The heaviest elements have so many protons in their nuclei that they can attract electrons with such force that the latter are accelerated to relativistic speeds,” says Folden. “This could affect the periodic aspect of the periodic table.”
As electrons approach the speed of light, he adds, orbitals with significant electron density near the nucleus (like s and p orbitals) are contracted and stabilized, and other orbitals (like d and f orbitals) are expanded and destabilized. Orbital contractions and expansions similar to these are the reason mercury is a liquid at room temperature and gold is yellow instead of silvery like other metals.
Because of these relativistic effects, Folden says, some scientists predicted that the d orbitals in element 112, copernicium, would act like valence orbitals. On the periodic table, copernicium sits just below mercury, cadmium, and zinc--all transition metals. On the basis of its location, you would expect it to behave like a transition metal. But these relativistic effects on its d orbitals indicated that copernicium might instead behave like a noble gas. In 2008, a JINR team was able to test a sample of copernicium. “Based on the available data, it looks like copernicium is more like a transition metal after all,” says Folden. “But this is an example of how the periodicity of the table might fail,” he explains.
Getting into Superheavy Element Research
With the island of stability on the horizon, there are magic numbers still to be determined, and while the limits of the periodic table are being probed, there are a lot of questions for new chemists to pursue. Shaughnessy encourages interested students to study “nuclear physics or physical chemistry and then find internship opportunities to get on-the-job training. Both nuclear chemistry and radiochemistry are important to the national laboratories, and there are many opportunities for someone willing to put in the extra work to develop a background in either field.”
Folden agrees. “The biggest hurdle for most chemistry majors is that very few universities offer undergraduate courses in nuclear chemistry.”
Students can supplement their studies with online resources and hands-on workshops, Folden adds. “I am affiliated with the [ACS Division of Nuclear Chemistry] Nuclear Chemistry Summer School, which is a great opportunity. There are also Research Experience for Undergraduates programs in nuclear science at several universities, including Texas A&M. Few students enter these programs with previous experience, so we will teach you what you need to know.”
Although progress has been made in understanding massive nuclei, deep questions about these fundamental building blocks of our universe remain unanswered. Whether the answers come today or tomorrow, one thing is for certain: There’s plenty for new nuclear chemists to explore!

References
Discovery of Elements 113 and 115. Lawrence Livermore National Laboratory. Retrieved Feb. 24, 2019, from https://pls.llnl.gov/research-and-development/nuclear-science/project-highlights/livermorium/elements-113-and-115.
Element 114—Superheavy Element Puts LLNL on the Periodic Table. Lawrence Livermore National Laboratory. Retrieved Feb. 24, 2019, from https://pls.llnl.gov/research-and-development/nuclear-science/project-highlights/livermorium/elements-114-and-116.
Folden, C. (2019). The Next Element: How Chemists are Expanding the Periodic Table. In ACS Webinars. American Chemical Society. www.acs.org/content/acs/en/acs-webinars/popular-chemistry/heavy-elements/video.html?sc=190523_webinar_popchem_em_eb_iypt.
Kragh, H. From Transuranic to Superheavy Elements: A Story of Dispute and Creation. Cham, Switzerland: Springer, 2018.
Oganessian, Y. T.; Rykaczewski, K. P. A beachhead on the island of stability. Physics Today, 2015, 68(8), 32–38. https://doi.org/10.1063/pt.3.2880.