Accidental Synthesis Yields New Catalyst for Cleaner Drinking Water

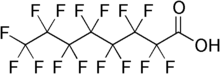
Perfluorooctanoic acid (PFOA) is a surfactant used to manufacture a variety of products, such as waterproofers, carpet cleaners, floor wax, and sealants. The problem with PFOA and similar compounds is that they may threaten human health. Chronic exposure at the parts per trillion is correlated with increased of cancer and damage to the liver, reproduction, development, and the immune system. Although PFOAs are being phased out, they continue to contaminate drinking water in many parts of the United States and recently triggered a state of emergency in Michigan.
It is not easy to remove these chemicals from water. With strong carbon-fluorine bonds, they are “among the most recalcitrant pollutants ever produced,” says Timothy Strathmann, an environmental engineer at Colorado School of Mines, who was not involved in developing the new catalyst. Granular activated carbon is often used to pull perfluorinated molecules out of water, but this process is inefficient because naturally present organic matter also sticks irreversibly to the carbon, using up a capacity to grab the pollutants.
Ezra L. Cates of Clemson University is one of many scientists investigating photocatalysts that are excited by UV radiation and degrade the contaminants into harmless compounds. Cates and his colleagues made an accidental breakthrough while testing the photocatalyst bismuth phosphate (BiPO4). After finding that it could break down PFOA effectively, they tried to improve its performance by varying the pH of the reaction used to make the catalyst particles. The researchers had hoped this would change the particles’ size and shape, but the process inadvertently produced bismuth oxyhydroxyphosphate (BOHP, Bi3O(OH)(PO4)2). This new catalyst has the highest photocatalytic activity for PFOA degradation ever reported (Environ. Sci. Technol. Lett. 2018, DOI: 10.1021/acs.estlett.8b00395).

In lab tests under UV light, BOHP degraded all the PFOA in a 130 μM solution in about one hour, roughly 15 times faster than bismuth phosphate or the current standard, gallium oxide (GaO). BOHP performed almost as well over five repetitions of the experiment, suggesting it can be reused.
To simulate PFOA-contaminated groundwater, the researchers then tested a more dilute solution of 1.2 μM PFOA accompanied by 500 μg dissolved organic carbon per liter. In this case, the photocatalyst also degraded all of the PFOA, though it took two hours. Strathmann calls it a “promising development” that advances the application of photocatalytic treatment of PFOA.
Cates suggests that BOHP degrades PFOA so quickly because its beefed-up hydroxide content gives it a highly positively charged surface at the low pH of the experiments compared with bismuth phosphate. PFOA has a pKa of around 0, dissociating easily in solution to its conjugate base perfluorooctanoate and H+. The surface of the catalyst attracts the negatively-charged perfluorooctanoate and decomposes it.
By monitoring some of the products of the reaction, the team found that 63-84% of the PFOA was converted into benign products, such as fluoride ions and carbon dioxide. Cates says they will soon be analyzing the remaining products to determine whether shorter-chain perfluorinated compounds remain. He and his team are now working with the company Purifics to carry out pilot-scale tests of the catalyst on groundwater samples from military sites contaminated by firefighting foam.
This story is adapted from “Photocatalyst shreds drinking water contaminant PFOA: A new bismuth-based catalyst is the fastest photocatalyst yet to degrade the perfluorinated pollutant.” D. Lockwood. Chemical & Engineering News. 96(36), September 6, 2018.