Assembling the Modern Periodic Table
By Julianna Poole-Sawyer
The periodic table is an elegant demonstration of properties of elements. You can determine the electron configuration of any atom, simply from its place. You can compare electronegativity, ionization energy, atomic radius, chemical reactivity, and more.
If I gave you all of the elements on cards and told you to recreate the periodic table, you probably wouldn’t have much trouble. You would order them by increasing atomic number and create a new row when you hit a noble gas. If you know about atomic numbers and electron shells, recreating the periodic table is simple. However, the periodic table predates knowledge of atomic numbers and subatomic particles (yes, including electrons). It even predates knowledge of the noble gases.
So how did Russian chemist Dmitri Ivanovich Mendeleev and the other creators of the periodic table (arguably six of them) bring order to the elements? How did they create a tool that would ultimately house 118 elements when they knew only 62 of them? And why does Mendeleev get all the credit?
Mass + reactions = periodic table
The modern periodic table didn’t spring fully formed from the genius of Mendeleev; it was shaped by key discoveries about the elements. One such discovery was that of atomic masses. Here is where we will begin our journey to periodicity. The modern definition of atomic mass (the weighted average of the atomic masses of all isotopes of an element) was meaningless 150 years ago. Chemists didn’t know about isotopes. In fact, many chemists held the view that atoms were the smallest units of matter possible. It would be 30 years after Mendeleev’s periodic table that scientists found out atoms were composed of smaller bits and pieces. The idea of isotopes wasn’t introduced until 1913, and neutrons weren’t discovered until 1932.
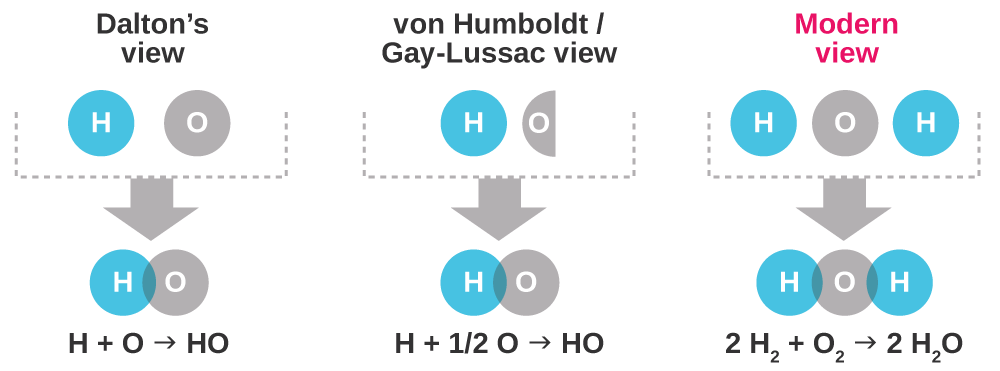
So how did chemists of the 19th century define atomic mass? In 1803, English scientist John Dalton published an article in which he assigned hydrogen a weight of 1, and then used compounds of hydrogen to determine the relative weights of the other elements. For example, to determine the atomic mass of oxygen, he used the fact that 1 gram of hydrogen reacts with 8 grams of oxygen to make water. He then could use this ratio of 8:1 to determine the weight of oxygen compared with that of hydrogen.
The problem with this method is that Dalton didn’t know the numbers of oxygen atoms and hydrogen atoms in a water molecule; he assumed water had a molecular formula of HO, leading to an incorrect relative atomic mass of 8 for oxygen.
To add even more confusion, in 1805 Prussian scientist Alexander von Humboldt and French scientist Joseph Louis Gay-Lussac determined that two volumes of gaseous hydrogen always combined with one volume of gaseous oxygen to form two volumes
of water vapor. The pair found numerous other simple ratios, resulting in Gay-Lussac suggesting that equal volumes of gases have equal numbers of particles, what we now refer to as Avogadro’s law. The problem with this hypothesis was that for it to be true, somehow the gaseous oxygen had to be splitting in half. Many chemists, including Dalton, considered this possibility absurd: how could an atom—at the time believed to be the smallest unit of matter—split during the course of a chemical reaction?
The mystery was solved in 1811 by Italian scientist Amedeo Carlo Avogadro, who argued that gaseous oxygen is composed not of atoms of oxygen but of molecules of oxygen: O2. Unfortunately, although he was an accomplished scientist, Avogadro was not an accomplished writer, and his hypothesis was not accepted for another 50 years.
The 1860s: a turning point
In September 1860, chemists from all over Europe met in Karlsruhe, Germany, for a conference of lasting importance. The goal was to systematize chemistry to choose strict definitions for terms such as “molecule” and “atom”. At the conference, Italian chemist Stanislao Cannizzaro persuasively presented Avogadro’s hypothesis of diatomic molecules and all of their implications for molecular formulas and accurate determinations of atomic masses. Cannizzaro’s work left a palpable impression on two chemists in attendance: Julius Lothar Meyer and Mendeleev. A mere 22 years later, these men were jointly awarded the Royal Society’s Davy Medal for the periodic systems they developed.
After the Karlsruhe conference, explorations of elemental periodicity exploded. Six different scientists, nearly simultaneously, took a hand in organizing the elements in the 1860s: Alexandre-Émile Béguyer de Chancourtois (1862), John Newlands (1863), William Odling (1864), Meyer (1864), Gustavus Detlef Hinrichs (1867), and Mendeleev (1869). Let’s explore three journeys, of the most well known: de Chancourtois, Meyer, and Mendeleev.
The telluric screw
The first designer of the periodic table wasn’t a chemist at all; he was a geologist and adept at systematizing. French scientist de Chancourtois had previously tried his hand at organizing minerals, geology, geography, and even language, creating a universal alphabet. In the 1860s, he turned his attention to the elements.
In 1862, de Chancourtois presented his periodic ordering of the elements to the Académie des Sciences in Paris, and he published his table in a paper in the journal Comptes Rendus de l’Académie des Sciences.
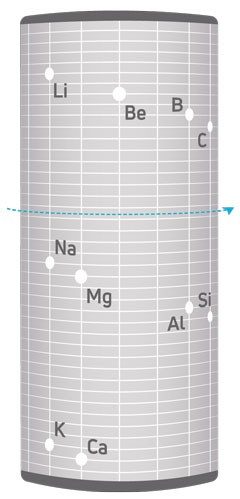
Early efforts to organize the elements had focused on triads, with scientists going out of their way to arrange metals in groups of three. But de Chancourtois’s system was a three-dimensional cylinder with the elements wrapping around it in order of atomic mass. This organization resembled a screw, with the elements on the threads. The element tellurium sat at the halfway mark; therefore, de Chancourtois called his system the telluric screw.
The elements weren’t just ordered from lightest to heaviest, however. With each turn of the screw, elements with similar properties aligned vertically: lithium was in line with sodium and potassium, magnesium was in line with calcium, and fluorine was in line with chlorine, thus showing periodicity of chemical properties.
At the time of its publication, the telluric screw received little attention from scientists because the journal Comptes Rendus did not publish de Chancourtois’s diagram of his system with his article, leaving an already complicated three-dimensional system explained only in words.
De Chancourtois’s telluric screw also contained some peculiarities, which probably did not encourage acceptance of the system. First, many of the elements didn’t line up according to their properties. For example, bromine isn’t in line with chlorine and fluorine and instead is in line with copper and phosphorus. Second, de Chancourtois included some other chemicals besides the elements, such as some compounds and alloys.
Despite this, de Chancourtois was the first to state that chemical properties correlate with atomic masses. In his article, he stated, “The properties of bodies are the properties of numbers.”
The facts in two dimensions
The next scientist of mention on our road to periodicity is German chemist Meyer. Meyer’s epiphany occurred at the Karlsruhe conference when he learned of the work on atomic masses by Cannizzaro. He wrote that when he read Cannizzaro’s article, “the scales fell from my eyes and my doubts disappeared and were replaced by a feeling of quiet certainty.”
Meyer’s breakthrough was presented in his textbook Modern Theories of Chemistry and Their Significance for Chemical Statics in 1872. In his table, Meyer organized the elements according to their atomic masses and valences, the latter of which had been discovered in the 1850s.
Meyer accounted for two important features that are usually attributed only to Mendeleev: he reversed the order of tellurium and iodine, and he left gaps. Without atomic numbers, the placement of tellurium (atomic number of 52) and iodine (atomic number of 53) in the periodic table can be confusing. In order of increasing atomic mass, iodine, with a weight of 126.9 amu, should come before tellurium, with a mass of 127.6 amu, except that such an ordering doesn’t make sense when you consider their properties. Iodine is chemically more like chlorine and bromine, whereas tellurium is chemically more like selenium and sulfur. In constructing his table, Meyer decided that properties should override masses, and he put tellurium before iodine.
The second distinguishing characteristic of Meyer’s table is that he left gaps in it. Other scientists of the day tried to eliminate gaps in their tables, often by forcing elements into illusionary categories, but Meyer simply left blank spots in his. While he didn’t go so far as to predict the properties of then-undiscovered elements, he left a gap between silicon and tin, for example, that would later be filled by germanium.
Interestingly, Meyer regarded periodicity and the similarities among elements in groups as evidence that elements were composed of smaller, more fundamental particles, an idea that Mendeleev himself never accepted.
| Meyer’s arrangement of the elements was based on how they reacted (valence): | |||
| 4 werthig | 3 werthig | 2 werthig | 1 werthig |
| Si (28.1) | P (31.0) | S (32.1) | Cl (35.5) |
| Unknown element | As (74.9) | Se (79.0) | Br (79.9) |
| Sn (118.7) | Sb (121.8) | Te (127.6) | J (126.9)* |
| Other researchers would order the elements by atomic mass, not reactivity: | |||
| Si (28.1) | P (31.0) | S (32.1) | Cl (35.5) |
| Unknown element | As (74.9) | Se (79.0) | Br (79.9) |
| Sn (118.7) | Sb (121.8) | I (126.9) | Te (127.6) |
Werthig is valence. The valency of an element was originally a measure of its combining power with other atoms when it forms chemical compounds or molecules. The concept of valence developed in the second half of the 19th century and helped successfully explain the molecular structure of inorganic and organic compounds.
*"J" in the table above is for iodine.
Putting it all together
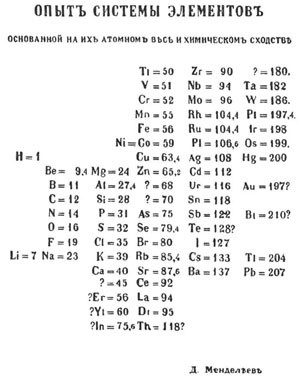
In February 1869, while writing the second volume of his chemistry textbook Principles of Chemistry, Mendeleev devised his own form of the periodic table. Popular accounts tell of Mendeleev shuffling and rearranging cards labeled with the elements and their properties, like a game of solitaire. Although historians have found no cards in Mendeleev’s archive, they have found myriad groupings of the elements, covered with scratched-out ideas and rearrangements. This work culminated in Mendeleev’s table in which he organized the elements by increasing atomic mass and aligned elements with similar properties in rows. In 1869, Mendeleev printed 200 copies of his table and sent them to colleagues throughout Russia and Europe.
Mendeleev went beyond just creating a table, however; he argued that the organization of elements reflected an underlying periodic law. For example, while Meyer switched the placement of tellurium and iodine, Mendeleev switched them and argued that the atomic mass of one of them had to be wrong. (The atomic masses were not, in fact, wrong, because periodicity turns out to be based on atomic number, not atomic mass.) Mendeleev corrected the masses of several elements on the basis of his table, and these corrections were later experimentally validated.
While Meyer left gaps in his table, Mendeleev predicted that elements would be discovered that would fill those gaps. He went so far as to predict their atomic masses and properties, and he named them: eka-boron, eka-aluminum, eka-manganese, and eka-silicon (“eka” is Sanskrit for “single” or "one"). This was a bold move; chemists at the time were expected to be reporters of existing facts, not speculators on what might yet be discovered. Although he wasn’t correct about all of their properties, when germanium, gallium, and scandium were discovered, chemists could see how they fit into the gaps of Mendeleev’s table, providing further validation for Mendeleev’s periodic law.
Mendeleev’s position as the father of the periodic table was solidified in the 1890s with the discovery of noble gases. At the time, not only was it inconceivable that an element could be nonreactive, but there was no room for them in the periodic table. In 1894, argon was discovered by British scientist Lord Rayleigh and Scottish scientist William Ramsay. When the only proposed noble gas was argon, Mendeleev and other chemists argued that it was not a new element but triatomic nitrogen (N3). After the discovery of helium, krypton, neon, and xenon, however, these inert gases couldn't be explained away. It wasn’t until 1900 that Ramsay suggested the new elements be given their own group between the halogens and alkali metals. Mendeleev responded thus: “This was extremely important for [Ramsay] as an affirmation of the position of the newly discovered elements, and for me as a glorious confirmation of the general applicability of the periodic law.”
The road to our modern-day periodic table was winding, full of dead ends and wrong turns. It required numerous discoveries, scientists, and experiments, as well as numerous failures and triumphs. It was, essentially, typical of science. Although we like to think of science evolving through lone geniuses like Mendeleev vaulting us toward progress, the reality of science is that it’s messy, requires extensive collaboration, builds on the work of others, and revises hypotheses when new information comes to light. Mendeleev, Meyer, and the others were indeed incredible scientists, not because they figured everything out themselves, but because they were fully enmeshed in the illustrious enterprise we call science.
About the Author
Julianna Poole-Sawyer studied molecular biology at Princeton University and the history and philosophy of science at the University of Notre Dame. She is currently a technical editor at Chemical Abstracts Service and a freelance writer based in Columbus, OH.
References:
Akeroyd, F. M. (2003). Prediction and the Periodic Table : A Response to Scerri and Worrall. J. Gen. Philos. Sci., 34(2), 337–355. Gordin, M. D. (2004). A Well-Ordered Thing: Dmitrii Mendeleev and the Shadow of the Periodic Table. New York: Basic Books. Scerri, E. R. (2007). The Periodic Table: Its Story and Its Significance. Oxford: Oxford University Press. Scerri, E. R.; Worrall, J. (2001). Prediction and the Periodic Table. Stud. Hist. Philos. Sci. A., 32(3), 407–452. https://doi.org/10.1016/S0039-3681(01)00023-1