Why Do Leaves Change Color in the Fall?

The equinox has passed, the temperature has dropped (in some places, anyway), and fall is truly here. Fall is an explosion for the senses, notably the vivid colors of autumn leaves. Chemistry happens right before our eyes as leaves change from bright green to brilliant shades of red, orange, and yellow. So what exactly is causing these changes? Read on to find out.
Hiding out in the green
The variety of colors uniquely observed during the fall season are actually embedded in leaves all year. The reason we don’t see them all the time is because chloroplasts in trees produce so much chlorophyll in the summer that green overpowers the other colors.
Chloroplasts are organelles in leaf cells that are responsible for photosynthesis, which provides the energy trees need to grow and reproduce. In plants, photosynthesis relies on chlorophyll, manufactured in abundance during the sun-intensive spring and summer. Chlorophyll absorbs red, orange, and blue light from the sun but hardly any green, which is reflected back to our eyes. Because of an ample amount of chlorophyll in trees during the spring and summer, the leaves appear green, and the underlying colors of the leaves are hidden.
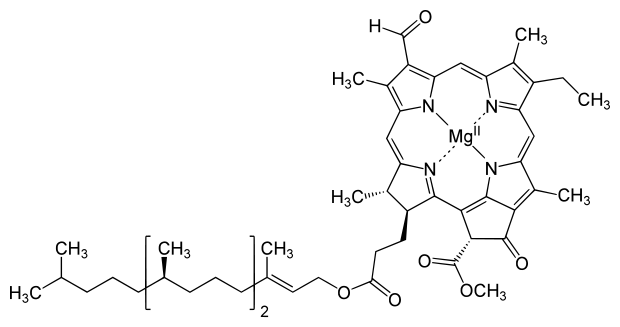
A key feature of chlorophyll is four pyrrole-like rings joined into a larger ring that form a porphyrin structure. Porphyrins are often found in brightly colored biological molecules, most notably the bright-red hemoglobin in your blood. Who knew blood and leaves had so much in common? The difference in color comes from metals the porphyrins in chlorophyll bind magnesium, whereas the porphyrins in hemoglobin bind iron. Also, the two molecules have different organic components bound to the porphyrins, which changes their appearance and reactivity.
Chlorphyll’s absorption of solar energy sets off a series of redox (electron transfer) reactions that convert carbon dioxide and water into glucose and oxygen. The plant stores some of the glucose and uses the rest to grow, and it releases the oxygen into the atmosphere. The redox reactions also break down the chlorophyll, which means the plant must constantly rebuild it to survive.
When bold colors emerge
As the days shorten in the fall, the trees and shrubs that give a spectacular visual show prepare for winter by shutting down their energy-intensive chlorophyll production and instead rely on stored glucose for energy. (Shedding their leaves also allows trees to conserve the water that would otherwise evaporate off of those broad, flat surfaces.) In this process, the chlorophyll is slowly broken down by the plant, diminishing the green color while exposing colors associated with other compounds present in the leaves.
Two categories of compounds produce the bright colors of fall: carotenoids and flavonoids. Carotenoids are present in tissues or cells of animals or plants. Plants use these molecules to assist chlorophyll in the absorption of light and to help protect the chlorophyll from solar radiation damage. Carotenoids can also act as antioxidants, which help protect energy-producing structures in plant cells. Common carotenoids found in leaves include lutein and beta-carotene.
The yellows are attributed to a class of carotenoids called xanthophylls and two types of flavonoids called flavonol and flavone. The orange can come from the carotenoid beta-carotene, which strongly absorbs green and blue light and reflects red and yellow. This allows our eyes to perceive the orange color. Violaxanthin is another carotenoid responsible for orange coloration of leaves.
Flavonoids have a different structure from carotenoids and typically have more phenyl rings in the molecule. Flavonoids likely contain more oxygen atoms as part of their structures as well. Although flavonoids are found in leaves, they are essential in the coloration of flowers. Additionally, they play a role in ultraviolet absorption and energy production.
The brilliant reds and purples of some leaves come from anthocyanins. Unlike the other colorful compounds, anthocyanins are only produced in the fall. They are the product of complex reactions among the plant’s glucose, phosphate, and other factors. So, the more glucose a tree has stored, the more anthocyanins it can produce, making for brighter displays. Additionally, color is pH-dependent, so soil acidity can play a role in leaf color.
There are a couple of reasons why plants might use some of their precious glucose to make anthocyanins when they could be storing it up for winter. Plants use the anthocyanins to attract animals to the fruits, which in turn helps the plant distribute seeds. Trees that are exposed to more sunlight produce more anthocyanins. It is thought that anthocyanins may also act as photo-inhibitors, or “sunscreens”, to protect the plant’s cells from potential damage from high-energy ultraviolet light from the sun at a time when chlorophyll isn’t around to do the job.
Brown colors come from tannins, which are also always present in plants. They help protect the plant from predators and may have a role in growth.
Why sometimes it is easier to be green
Of course, not all trees lose their green color in the winter. Conifers and many tropical plants are evergreen, retaining their chlorophyll supply year-round.
In the case of tropical plants, the reason is simple—they thrive in temperate climates closer to the equator. There, sun and unfrozen water are plentiful throughout the year, so the plants have no need to shut down chlorophyll production or shed healthy leaves.
Conifers, such as pine, fir, and spruce, are another story. These plants have acclimated to tougher conditions, where water and nutrients are scarce and colder temperatures are plentiful. Under these conditions, it is tough to store enough glucose to survive the long winter.
Fortunately, conifers have a number of traits that enable them to work their chlorophyll-powered factories all year long. Their leaves take the shape of long, thin needles, which are coated with cutin—a waxy polyester. The thin shape and waxy coating help prevent water evaporation, so photosynthesis can continue.
Additionally, many evergreens produce their own antifreeze. While glucose, like any solute, depresses the freezing point of water in the tree, conifers also produce antifreezing hormones (such as jasmonic acid) and proteins. Antifreezes are important to the health of the tree; when water freezes, the molecules take on a hexagonal configuration that occupies more space than liquid water. In other words, water expands when it freezes, which would burst the cell walls and kill the plant. (This also way vegetables that aren’t frozen properly get mushy.) So, the antifreeze keeps the water available for photosynthesis and the trees’ cell walls in tact.
Green, yellow, organge, red, purple, and brown. The chemistry of changing leaves produces a feast of color for the eyes!