Gold Nanoparticle Sensor Produces Simple Urine Test for Cancer
by Giuliana Viglione, for C&EN
Detecting some cancers early can improve a patient’s chance of survival, but conventional detection methods can involve costly tests and equipment, making them inaccessible for people in low-resource areas. Now, researchers have developed a diagnostic test that can detect a molecular signature of cancer and other diseases with a simple readout: it turns urine blue (Nat. Nanotechol. 2019, DOI: 10.1038/s41565-019-0527-6).
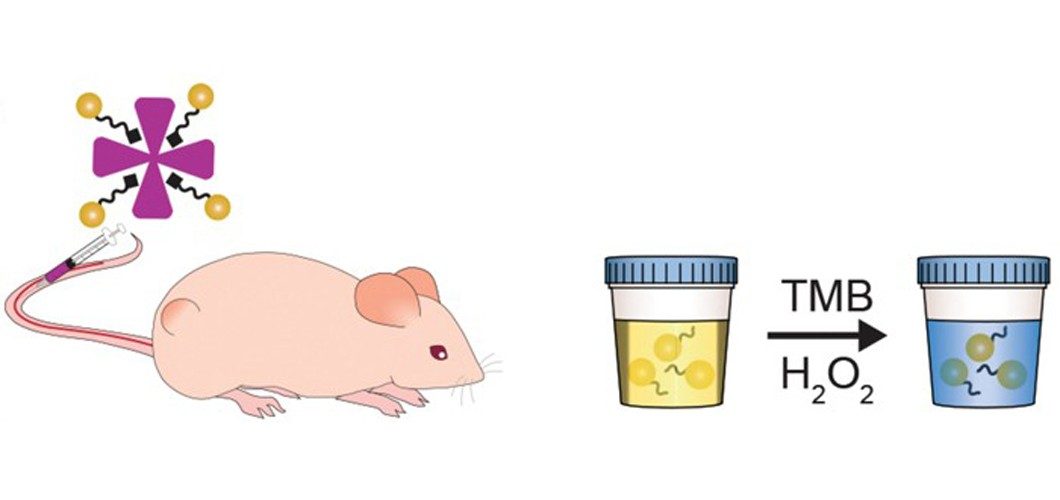
Imperial College London biomedical materials scientist Molly Stevens teamed up with Massachusetts Institute of Technology biomedical engineer Sangeeta Bhatia to develop the approach, which they think has the potential to help patients in low-resource and rural areas, where available medical technology may be limited. Stevens specializes in low-cost catalyst-based diagnostics and Bhatia works on creating nanosensors that respond to enzymatic activity. The two combined their expertise to create nanoparticle-protein complexes that, once injected, can reveal the presence of disease-related enzymes through a simple urine test.
The team synthesized the complexes by using peptides to link gold nanoclusters to neutravidin, a protein carrier. They designed the protein linkers so that enzymes called matrix metalloproteinases (MMPs) can cleave them. Certain types of tumors, including colorectal cancer, release these enzymes, and MMPs have previously been found to be a potential prognostic for several cancers.
To test their sensors, the team injected the complexes into healthy mice and those that had received colorectal cancer grafts. In healthy mice, the complexes remained intact and were therefore too large to quickly pass through the renal system to end up in the animals’ urine. However, in the tumor-bearing mice, the tumors’ metalloproteinases severed the gold nanoclusters from the carrier proteins and the mice could excrete the particles. To detect the presence of the particles in a urine sample, the scientists treated it with hydrogen peroxide and 3,3’,5,5’-tetramethylbenzidine (TMB). The gold particles catalyzed a reaction between TMB and the peroxide to produce a blue compound. Within an hour of injecting the complex in a tumor-bearing mouse, its urine turns blue, a color change that is highly visible to the naked eye.
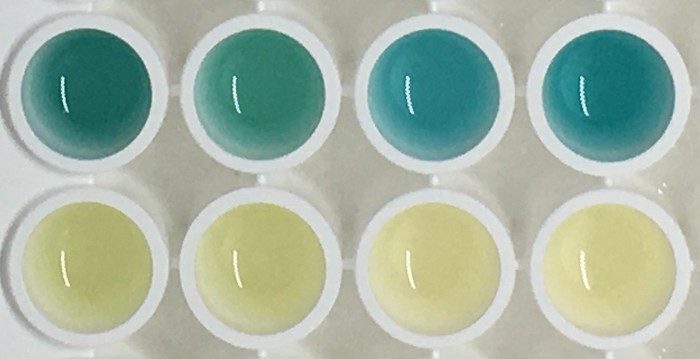
The team found no toxicity related to the injected complexes, and both the complexes and the cleaved gold nanoparticles were undetectable in the mice within 4 weeks of injection, suggesting that the body eventually does clear them.
Since enzymes “play active and functional roles” in many cancers and infectious diseases, Stevens says, applying this approach to other types of disease is a matter of designing a sensor that will be cut by enzymes specific to those diseases. She’s particularly interested in developing these sensors for detecting drug-resistant microbial infections.
Rajesh Sardar, an analytical chemist at Indiana University–Purdue University Indianapolis, says the study was a “very well-thought and well-planned” proof of concept, providing a pathway towards building a potential nanoparticle-based system for detecting disease. However, Sardar cautions, the human body is a much more physiologically complex environment than a mouse’s, and there is still much “room to optimize the system” before such technologies could be tested in human subjects.