How Hair Removers Get Rid of Unwanted Fuzz
Summer is in full swing in the U.S. Northern Hemisphere. Temperatures are rising, swimming pools are open for business, and bare arms and legs are emerging from winter clothing.
For some people, the start of shorts-and-swimsuit season means it’s time to get rid of body hair. These sun-seekers have an array of techniques to turn to: hair removal creams, waxing and sugaring, lasers, and even a good ol’ pair of tweezers. Knowing how the various options work might help you decide which one to use.
The way it was
Getting rid of body hair is a billion-dollar industry and growing worldwide. But depilatories are nothing new. Evidence indicates that prehistoric cultures used stones and shark teeth as crude razors. Some ancient Egyptians removed hair with tweezers, razors, and pumice stones and ripped it out with wax or sugar pastes, leaving behind only their eyebrows.
Chemical removers have ancient roots too. People living 6,000 to 7,000 years ago in what is now Turkey used hair removal creams made with quicklime (calcium oxide). Likewise, American Indians applied lye to get rid of their body hair, a practice colonists adopted. Bases like lye and quicklime hydrolyze nitrogen-containing amide bonds in hair’s proteins, breaking bristles down. Drain-clog removers work the same way.
Not every historical hair removal tactic has survived, and for good reason. Inventor Albert C. Geyser successfully marketed a machine in the first half of the 20th century that could permanently remove hair with a dose of X-rays. It was quickly banned once the detrimental health effects of the radiation became apparent. A study in the 1970s attributed fully one-third of radiation-related cancers in women to devices like Geyser’s.
Modern hair removal creams
Today, the hair removal options available at home or at professional salons are safe and—depending on your tolerance—relatively painless.
Creams are a popular option for at-home removal. To understand how these work, you first have to understand hair's composition. Hair is made of fibrous proteins called keratin, twisted like yarn or rope into long bundles. Keratin strands are cross-linked by covalent disulfide bonds and weaker hydrogen bonds. These are depilatory creams’ targets.
The active ingredients in brands such as Veet and Nair are salts of thioglycolic acid, usually potassium or calcium thioglycolate, in combination with bases such as calcium, sodium, or potassium hydroxide. (Yes, this is the same thioglycolate that—as an ammonium salt—is used in hair perms, and for much the same reason. Keep reading.)
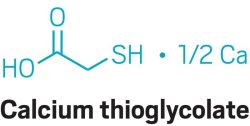
The bases serve two purposes. They cause the hair to swell, opening its keratin fibers to allow thioglycolate to penetrate. The bases also break the S-H bond on thioglycolate’s thiol group. Once thioglycolate’s proton leaves, its sulfur atom is free to attack the hair protein’s disulfide bonds. Break enough of those, and the hair degrades completely and can simply be wiped away.
Studies have shown that thioglycolate is remarkably selective for sulfur bonds. Researchers tested Nair on thin, thick, and medium hair, and on cotton, rayon, and polyester fibers. All three strands of hair broke within 10 minutes, but the remover had no effect on the other fibers, none of which contain disulfide bonds.
Other experiments have shown that cream hair removers should have a pH between about 12.0 and 12.5, ensuring that the products work quickly but aren’t so caustic that they burn the skin, which has a pH of 4.5–5.5. Dermatologist Meghan Feely says cream hair removers can still cause chemical burns for some people. They should be used according to their directions to minimize risk.
Because these chemicals are so effective, there is not much interest in finding new depilatory agents, says Heike Hanau, a marketing manager for Merck & Co., which used to supply calcium thioglycolate for hair removers. But she says chemists are still working to improve depilatories’ smell. One by-product of thioglycolate’s reaction with disulfide bonds is hydrogen sulfide, which smells like rotten eggs.
Waxing hair out

Waxing is another common method for hair removal that can be done at home or by a professional in a salon. Wax, a mixture of lipids and long alkanes, can come from bees, plants, or petroleum products. The long alkyl chains make the wax a malleable solid at or just above room temperature. For hair removal, the wax is generally heated and spread across the skin. As it cools and hardens, it traps hairs, and when it’s yanked away, it pulls them out or breaks them off.
Sugaring has emerged in recent years as a trendy alternative to waxing. It works by the same principle: Spread a thick paste across the skin, then pull it off, along with some hairs. Sugaring wax, as it's sometimes called, can be made at home with a recipe candymakers will recognize: Heat a mixture of water, table sugar, and lemon juice to about 120 °C until it turns golden brown, a process known as caramelization.
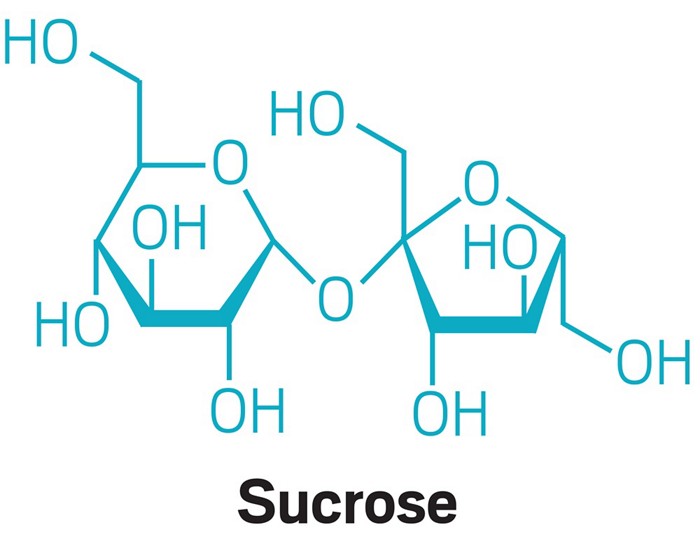
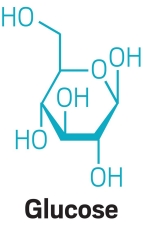
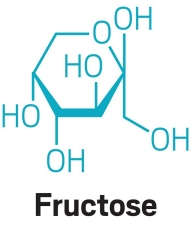
In this reaction, water hydrolyzes table sugar (aka, sucrose), splitting it into glucose and fructose. The acid in lemon juice acts as a catalyst by protonating the oxygen that links sucrose’s two halves, encouraging addition of a hydroxyl group from water. The product, the mixture of hydroxylated glucose and fructose, is known as invert sugar in the food world, and it crystallizes at a higher temperature than sucrose alone, making for a spreadable wax.
Whether sugaring is actually better than waxing is a matter of opinion. Fans of sugaring claim it penetrates more deeply into hair follicles for more complete removal. Society of Cosmetic Chemists President Perry Romanowski says there’s no evidence to support that claim. How well it works and how much it hurts mostly come down to the skill of the person pulling out the hair, he says. Sugaring aficionados also claim that the paste adheres only to dead skin cells, not live ones, reducing irritation when it’s yanked away. American University’s Matthew Hartings, who studies food chemistry and is also a member of C&EN’s advisory board, is doubtful. “I’ve got a lifetime of trying to clean caramel off my hands that calls shenanigans on that,” he says.
Laser hair removal

And then there are lasers, the newest entrant in the hair removal game. Professionals train these instruments, tuned to an infrared wavelength absorbed by the pigment melanin, on the hair that needs removing. Melanin gives hair—as well as skin—its color. The absorption heats up the hair, frying it down to its roots beneath the skin. It works best on dark hair against light skin, but experiments have shown that neodymium-doped yttrium aluminum garnet lasers, which can focus more tightly than the diode lasers commonly used, can be effective on dark skin.
Hair-removing lasers require approval by the U.S. Food & Drug Administration before people can use them. Other hair-removing products may also need federal approval if a bill introduced in the Senate becomes law. The Personal Care Products Safety Act would give FDA many of the same powers to regulate cosmetics that it currently has to regulate food. Companies would be required to disclose the ingredients in their products, and the legislation would give the agency jurisdiction to evaluate whether those ingredients are safe for people to use.
Hair removal has a long history and most of the methods around today have been used for decades or millennia, so depilatory methods are unlikely to change much. And ultimately whatever your personal choice may be to remove a little unwanted hair here or there is less important than just getting out there and enjoying the season!
This article is adapted from “What are hair removers, and how do they get rid of unwanted fuzz?" Chemical & Engineering News. 96 (22), May 22, 2018. https://cen.acs.org/business/consumer-products/hair-removers-rid-unwanted-fuzz/96/i22