2020 Nobel Prize in Chemistry Awarded for CRISPR Genome Editing
by Ryan Cross, C&EN

The 2020 Nobel Prize in Chemistry has gone to Emmanuelle Charpentier and Jennifer A. Doudna "for the development of a method for genome editing." That method, formally known as CRISPR/Cas9 gene editing, but often simply called CRISPR, allows scientists to precisely cut any strand of DNA they wish. In the 8 years since its creation, scientists have published thousands of experiments using CRISPR to alter DNA in organisms across the tree of life, including butterflies, mushrooms, tomatoes, and even humans.
CRISPR has been a boon for biologists who use the molecular scissors to probe the code of life in fundamental science experiments. But researchers have wasted no time in applying the tool to agriculture and human health. Several groups are using CRISPR to change the DNA of livestock and crops. Others are using CRISPR as the basis of one-time therapies that could potentially offer cures to genetic diseases like sickle cell disease and muscular dystrophy. CRISPR has even been used to make simple diagnostic tests during the coronavirus pandemic.
How does CRISPR work?
CRISPR-Cas9 combines a protein that can neatly snip DNA with a molecule that guides those molecular shears to a specific spot in an organism's genome. Watch this video to learn how it works.
Credit: Darren Weaver/Andrew Sobey/ACS Productions/C&EN
“The number of discoveries in biomedicine that have had the impact that Jennifer's and Emmanuelle's had can be counted on the fingers of one hand: recombinant DNA, PCR, DNA sequencing, and now CRISPR,” says Fyodor Urnov, a gene-editing scientist at the University of California, Berkeley. “We have never had a technology as powerful and versatile as genome editing with CRISPR, and it is a thrill to work with it daily.”
Charpentier, who is now at the Max Planck Institute for Infection Biology, and Doudna, at UC Berkeley, began working together in 2011. The two scientists were inspired by a strange, and little-studied bacterial immune system. It turns out that bacteria get viral infections just like people do, and some bacteria used an enzyme called Cas9 to chop up invading viruses and store molecular mugshots of them to quickly attack any repeat invaders. In 2011, Charpentier worked out the details of how two bacterial RNA molecules—called tracrRNA and crRNA—controlled this process (Nature 2011, DOI: 10.1038/nature09886).
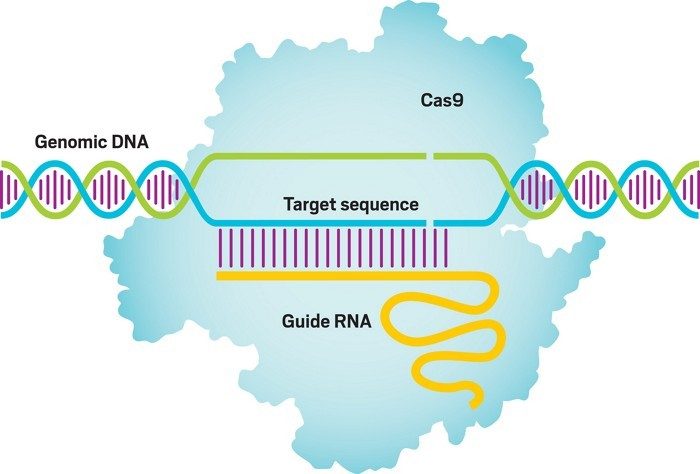
The two scientists began thinking about how they could retool this bacterial immune system into something that could be easily programmed for gene editing. They synthesized a new molecule called the single-guide RNA, which combines crucial features of the two bacterial RNAs to direct Cas9 to a specific site in DNA for cutting (Science 2012, DOI: 10.1126/science.1225829).
It was the tool that scientists had been waiting for. The method is cheaper, faster, and easier to use than previous gene-editing tools, which required complex and costly protein engineering every time scientists wanted to edit a new sequence of DNA. Other labs began adopting the technique within a year. Multiple companies dedicated to developing CRISPR gene-editing therapies for cancer and rare genetic diseases were founded within two years. Some of those firms count Charpentier and Doudna among their founders. Today, scientists can readily order Cas9 and guide RNAs customized to target a specific sequence of DNA. CRISPR opened up gene editing to the masses.
But CRISPR’s ease-of-access is a double-edged sword. Two years ago, Chinese scientist He Jiankui used CRISPR to edit two human embryos that were carried to term. The announcement of the first gene-edited babies—whose whereabouts and health are currently unknown—shocked the world. The experiment crossed an ethical red line in the minds of many gene-editing scientists, and brought new vigor to old debates about heritable gene editing. The Nobel committee did not wade deeply into these ethical quandaries but instead emphasized Charpentier and Doudna’s technological achievement and its potential to bring “the greatest benefit to humankind.”
“This is a well-deserved and predictable opportunity to celebrate CRISPR pioneers for the development of their revolutionary technology,” says Rodolphe Barrangou, a gene-editing scientist at North Carolina State University who began studying the CRISPR immune system in bacteria before it was conceived as a gene-editing tool.
“I think this is a very well selected prize,” says American Chemical Society (ACS) President Luis Echegoyen. Even though CRISPR’s applications are in the realm of biology, the technique “requires very sophisticated and very serious chemistry,” he says. “I think everyone has been waiting for CRISPR to be awarded the prize because the implications are truly amazing. Some things are so evident that they are going to have major implications for the future that you don’t need to wait around to figure out if this is really going to have an impact on humanity.” C&EN is published by ACS.
A Nobel Prize for CRISPR was highly anticipated, and many scientists speculated about who would be included, and who would be left out.
Surprisingly, the Nobel committee did not include Feng Zhang, a scientist at the Broad Institute of MIT and Harvard, in the award. Zhang is widely regarded as one of the inventors of CRISPR. His group independently described the creation of CRISPR gene editing in January 2013. It was the first demonstration of the tool in mammalian cells. But Zhang’s original paper relied on using the two bacterial RNA molecules—tracrRNA and crRNA—rather than the single guide RNA developed by Charpentier and Doudna, which is the version widely used by scientists today.
Another contender for a CRISPR Nobel Prize was Virginijus Siksnys of Vilnius University, who independently described CRISPR-Cas9’s potential for genome editing just months after Charpentier and Doudna. He was awarded the Kavli Prize in Nanoscience along with Charpentier and Doudna in 2018.
Charpentier and Doudna will equally share the prize money of roughly $1.1 million. Because of the coronavirus pandemic, the usual Nobel Prize ceremonies will be held virtually, with winners receiving their medals and diplomas in their home countries.
“It’s wonderful to see Jennifer and Emmanuelle recognized in this way,” says David Liu, a chemist at the Broad Institute, who has created new versions of CRISPR called base editors. “With new treatments for human genetic diseases already in patients, with early positive outcomes, the era of human genome editing has already begun, and Emmanuelle and Jennifer are two of the key pioneers responsible for initiating this new era.”