Novel Antibiotic Discovered in the Guts of Worms
by Alla Katsnelson, for C&EN

A newly discovered antibiotic compound, produced by bacteria living in the gut of a soil-burrowing parasitic nematode, slays some of the most nefarious superbugs (Nature 2019, DOI: 10.1038/s41586-019-1791-1).
The World Health Organization recently sounded the alarm about the need for new antibiotics that work against a large group of pathogens called gram-negative bacteria. A new class of such drugs has not been approved for about half a century, and these bugs are growing increasingly resistant to existing antibiotics. Gram-negative bacteria, a group of pathogens that include Escherichia coli, Pseudomonas aeruginosa, and Klebsiella pneumoniae, are especially difficult to target with new antibiotics because their dual outer membrane keeps drugs from penetrating.
Kim Lewis, who runs the Antimicrobial Discovery Center at Northeastern University, and his colleagues took their search for new antibiotics to an unusual place: the guts of a nematode. It’s common for bacteria to make compounds that target their rivals, but these natural compounds might be toxic or otherwise not work for people. The idea, Lewis says, was “to identify bacteria that have similar requirements for antibiotics” to humans. To fit the bill, a microbe should make compounds that kill gram-negative pathogens, are nontoxic to animals, and stick around in the body long enough to serve effectively as a medicine.
His lab focused on a bacterium called Photorhabdus, which lives in the gut microbiome of a nematode. Photorhabdus and its worm host collaborate to do a hit job on their prey. The nematodes infect insect larvae, then release the bacteria. Then the bacteria go in for the kill, spewing toxins. It’s also in Photorhabdus’ interest to protect its share of the meal. To prevent other bacteria present in the nematode gut from siphoning off the insect spoils, Photorhabdus produces compounds that kill rival bacteria, many of which are gram negative.
Some of those compounds, Lewis reasoned, might check all the boxes: they’re nontoxic to the nematode and they travel through the larval tissues, so their pharmacokinetics must be pretty good. “That’s where we thought the good antibiotics may be hiding—in the genomes of those bacteria—and that’s where we started looking very carefully,” says Lewis.
To identify promising compounds, the researchers grew large quantities of Photorhabdus in the lab, then concentrated their extract. They hit the jackpot when they identified a component of this substance, which they named darobactin, that kills E. coli.
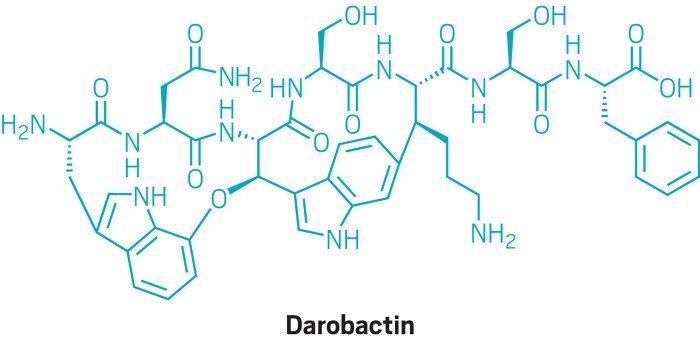
Darobactin has unusual chemistry. “It has two fused rings and one of them is made of an unactivated carbon-carbon bond,” says Lewis. “We have not seen that kind of a bond or a structure in antibiotics before.”
Darobactin is also unusually large for an antibiotic, clocking in at 965 Daltons. That’s too large to penetrate gram-negative bacterial membranes, so it wasn’t immediately clear how darobactin works. To find out, the researchers repeatedly exposed E. coli to the compound until resistant bacteria emerged. Those bacteria carried mutations in a gene coding for a molecule called BamA, which sits atop bacteria’s outer membrane.
“The target they identified, BamA, is interesting because it is an outer-membrane protein,” says Alexander Mankin, a biochemist at the University of Illinois at Chicago. “Therefore, darobactin does not need to get across the membrane. This is very cool.”
Darobactin kills several gram-negative bacteria in culture, including E. coli, Klebsiella, Shigella, and Salmonella. They also tested the compound in mice. A single dose protected mice infected with E. coli, K. pneumoniae and P. aeruginosa, with no toxicity, while untreated animals died within 24 hours.
The compound doesn’t inhibit the growth of all gram-negative bacteria at low concentrations, says Karen Bush, a biochemist at Indiana University. “However, darobactin may serve as novel scaffold” that medicinal chemists can tweak to make a more widely effective drug, she says.
Lewis and his team found the sequence for darobactin in multiple species of Photorhabdus, but also in other bacteria—including a species of Yersinia that lives in the human gut. They are continuing to mine the nematode bacteria for other medicinal treasure. “Photorhabdus has been around for 370 million years,” Lewis says. “During that time they probably screened the entire planet for antibiotics that are useful for them—and so, are useful to us.”




