Atomic News Roundup—June 2023
In this selection of research reported in Chemical & Engineering News, we highlight edible batteries, a new sorbent that collects high amounts of CO2, a 3-D capture of a smell receptor structure, and ancient mummifying ingredients.
Edible battery could power digestible devices
Edible sensors could someday travel through a patient’s body, sending critical information to medical providers along the way. With the development of a new, safe rechargeable battery made entirely from food ingredients and additives, the battery could power devices, be recharged dozens of times wirelessly, and last a couple of days in its journey through the gut.
Among other ingredients, the recipe called for the redox-active molecules riboflavin, also known as vitamin B-2, and quercetin, a flavonoid commonly found in plants and berries.
Mario Caironi and his colleagues at the Italian Institute of Technology made energy-generating reactions from sushi seaweed and edible foil. And they made electrodes by loading activated charcoal with riboflavin (aka, vitamin B-2) for the anode or quercetin.
Seaweed soaked in an electrolyte solution separated the electrodes while food-grade decorative gold collected the electrical current. A beeswax coating protected everything from stomach acid.
The edible battery generated a current of 48 µA for more than 12 minutes. The researchers are working to make it capsule-sized and test it with edible devices.
Read the full article in C&EN: An edible battery gets its juice from food
Sorbent exhibits record capacity for collecting CO2 from air
Removing CO₂ from the air could help tackle climate change. A new high-capacity sorbent shows promise for collecting large amounts of CO₂ and then converting it into baking soda for ocean storage.
To create the sorbent, Arup K. SenGupta and colleagues at Lehigh University sent a copper chloride solution through resin beads. The nitrogen atoms in the beads' polyamine groups bound the copper ions in the solution.
Next, the researchers passed air over the copper-loaded beads. Water vapor and CO₂ in the air formed bicarbonates. The copper ions bound them, thus removing CO₂ from the air. The sorbent collected more than 5 mol of CO₂/kg in ambient air—double to triple the capacity of rival sorbents.
As seawater passed through the beads, the copper ions released the bicarbonates to bind with chloride in the water. The resulting sodium bicarbonate, or baking soda, could be safely stored in oceans, the researchers say.
Read the full article in C&EN: New sorbent captures 3 times as much CO₂

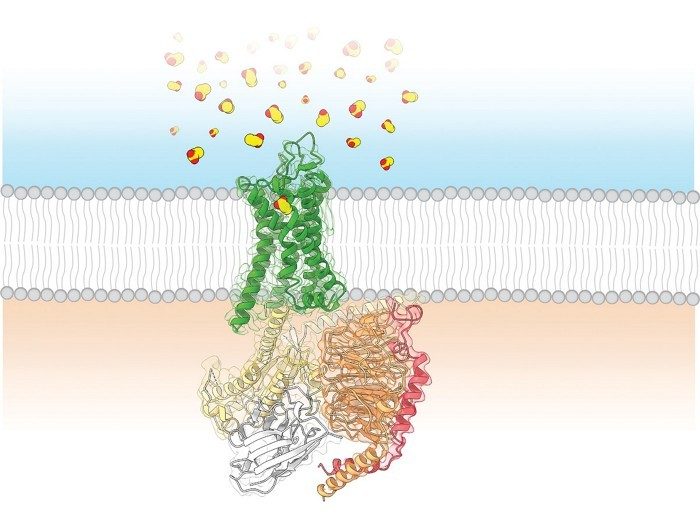
Researchers capture a smell receptor’s structure
For the first time, scientists have obtained the 3-D structure of a human olfactory receptor and discovered how it binds to an odorant. The results provide insight into how the 400 smell receptors in a human nose recognize aroma compounds.
Most smell receptors are unstable outside of the body, but researchers were able to use cryo-electron microscopy to resolve the structure of OR51E2, a receptor sensitive to water-soluble compounds. They then used computer simulations to determine how it binds to propionate, a key odorant in Swiss cheese known to activate the receptor.
Simulations of the chemical interactions between propionate and OR51E2 suggest that the odorant may activate the receptor by binding with the amino acid arginine.
Moving forward, the multi-institutional group led by Aashish Manglik at the University of California, San Francisco, plans to modify OR51E2 to predict the structures of other receptors.
Read the full article in C&EN: Scientists sniff out the structure of a human olfactory receptor
Chemical analysis reveals ingredients for mummifying a body
In 2018, archeologists discovered an ancient Egyptian embalming workshop. Inside were ceramic vessels that once held oils and balms for preparing bodies to be mummified—and many were labeled. Using chemical analysis, researchers have identified the contents of 31 of the most clearly labeled vessels.
Researchers at the National Research Centre of Cairo used gas chromatography/mass spectrometry (GC/MS) to analyze samples of the artifacts, which dated from the 6th and 7th centuries BCE. Various mixtures of plant oils and tars, resins, and animal fats were identified and compared with the labels for a clearer picture of the mummification process.
For example, a balm previously believed to be myrrh or frankincense has been found to be a mixture of cedar, juniper, and cypress oils, all of which have insecticidal and antimicrobial properties. Other discoveries highlight trade networks established through Africa and Southeast Asia.
Read the full article in C&EN: Ancient pots contain chemical secrets of mummification






