Flu Shots Aren’t Always Effective. Could Chicken Eggs be a Culprit?
By Leigh Krietsch Boerner, for C&EN
It’s one of the last things you want to see when you’re pregnant. My husband was shivering next to me in bed, his forehead burning. “I think you have the flu,” I told him. “I can’t,” he said. “I got a shot.”
But he did have the flu. And pretty soon, so did I.
It’s not uncommon for flu shots to be less effective than intended. The 2014–15 flu season, during which I got sick, was severe, according to the US Centers for Disease Control and Prevention (CDC). The CDC estimated that the flu sickened 40 million people and sent 970,000 to the hospital in that period. The flu vaccine was only 23% effective against the common influenza type A and B viruses that season. For the H3N2 virus, a subtype of influenza A, which was the most prevalent virus that circulated, the vaccine was just 13% effective.
The CDC attributed the low effectiveness to a phenomenon called drift. More than 80% of the circulating H3N2 viruses the CDC saw in patient samples in the 2014–15 season were different, or had “drifted,” from the viruses at which manufacturers had aimed their vaccines.
The tendency of a flu virus to evolve has to do with how it replicates. Influenza uses its own RNA polymerase enzymes to replicate, according to Matthew Miller, a virologist and immunologist at McMaster University. “This makes the virus particularly prone to errors,” he says, meaning that the new viruses produced during replication can look different to our immune systems than the original viruses.
Recent evidence has emerged that the efficacy of flu shots can also be affected by how the pharmaceutical industry makes its vaccines. Most flu shots are currently produced from viruses grown in chicken eggs containing an embryo. Scientists have observed that mutations can occur in influenza viruses grown inside such avian cells, potentially leading to vaccines that are less effective because they are aimed at a slightly different version of the virus than the one we’re exposed to. Not everyone believes that such mutations cause enough of a drop in vaccine effectiveness to justify abandoning egg-based vaccines, however, and they are calling for more studies.
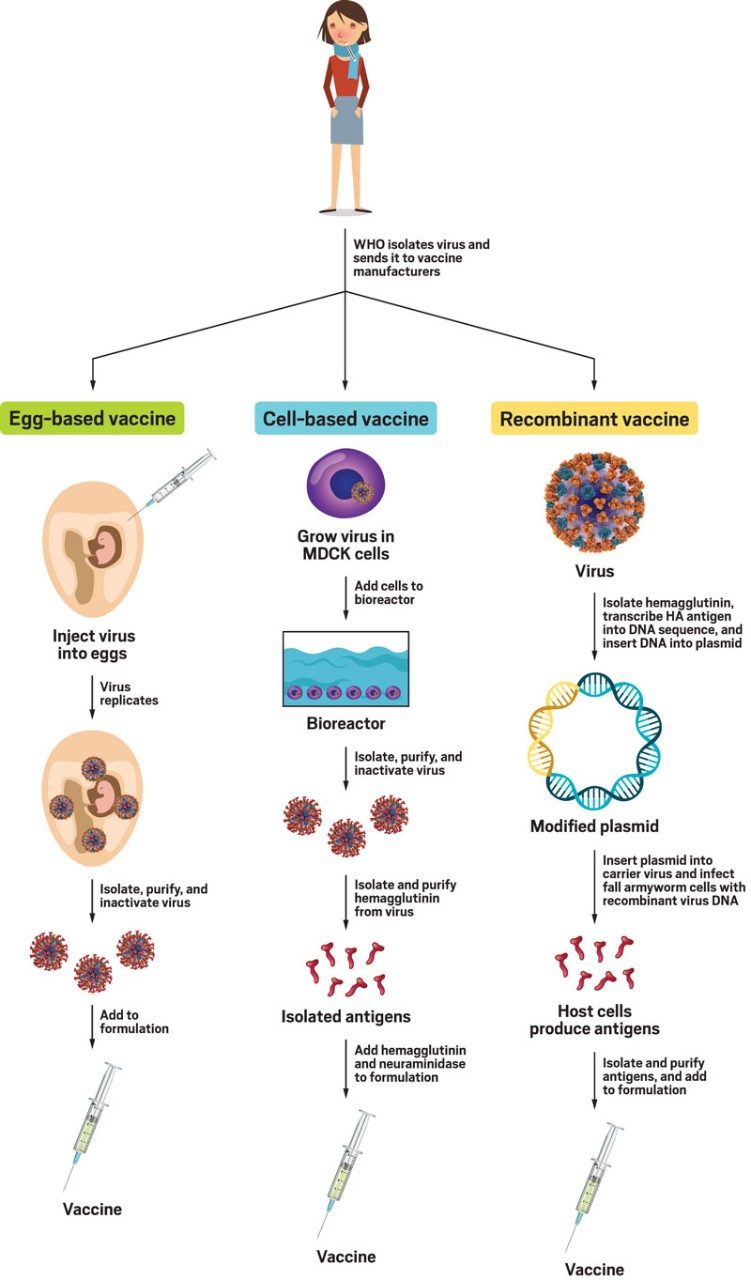
Making a flu vaccine
Vaccine makers produce flu shots in three ways.
Left: the egg-based pathway, how chicken-egg-based vaccines are made.
Middle: the cell-based pathway, how Flucelvax is made.
Right: the recombinant pathway, how Flublok is made.
Sources: Sanofi, Seqirus, Matthew Miller
Note: "MDCK cells" refer to Madin-Darby canine kidney cells.
To Egg or Not to Egg
The World Health Organization (WHO) is in charge of annually determining which viruses will be included in the flu vaccines administered by health-care workers. The WHO usually bases its choices for the Northern Hemisphere on the viruses that circulated in the Southern Hemisphere during its earlier flu season. It typically takes 5–7 months for the WHO to send out the viruses it has chosen and the vaccine industry to receive the shipment, replicate the viruses, and manufacture millions of vaccines. Sometimes the WHO guesses incorrectly about the viruses, which results in a less effective flu shot. But that 5-to-7-month lag also gives viruses time to drift. That means the viruses that end up in vaccines are different than what’s circulating, according to Anthony Fauci, the head of the National Institute of Allergy and Infectious Diseases (NIAID). Sometimes it’s a little different, but sometimes it’s a lot different.
“If you get it right from the beginning and it doesn’t change much, you get a relatively effective vaccine; if you get it wrong, you get a poorly effective vaccine,” Fauci says. “If you get it right but the virus changes a bit by the time the vaccine is ready for distribution, then you may get an intermediately effective vaccine,” he says.
But research shows that drift doesn’t occur only in replicating viruses in the “wild.” Given the chance, viruses will mutate anywhere—even as they’re replicating in the lab.
There are currently three types of influenza vaccines available: recombinant, cell based, and egg based. They all start with the same viruses from the WHO. Scientists at pharmaceutical companies receive only about a vial’s worth of the viruses from the organization, so the firms need to replicate them to create enough for millions of flu vaccines. The process is different for each type of vaccine.
Growing influenza viruses in eggs is the oldest way of making flu vaccines. Scientists inject a live virus into an embryonated egg, let the virus replicate, collect the replicates, purify them, and then kill them. They use those inactivated viruses to make the flu vaccine.
Influenza vaccines are generally made from inactivated flu viruses so that getting a flu shot won’t make a person sick, but the inactivated version can still jump-start the immune system. Flu viruses make antigens—toxins released by a virus—which cause an immune response. Sensing an antigen causes the body to produce antibodies—specific proteins made to fight a specific antigen. If the person later encounters that virus circulating in the wild, the antibodies will recognize the virus’s antigens and attack.
Some say that there’s a slight problem with making flu vaccines with eggs: avian cells, such as those in chicken eggs, are slightly different than human cells, notably in the molecules that coat their surfaces.
“The flu virus has two major proteins on the surface,” says Sarah Cobey, a computational evolutionary biologist at the University of Chicago. They are hemagglutinin and neuraminidase—the Hs and Ns, respectively, in flu viruses’ official names. Hemagglutinin is the more common protein and the one that the influenza virus uses to bind to a host cell’s receptors, Cobey says. Neuraminidase is responsible for busting open the influenza virus so it can enter the host cell. So from the virus’s perspective, H and N proteins are essential for replication. From our perspective, she says, “they are the major targets that we’re trying to train our immune system on.”
For the virus to replicate inside the chicken eggs, however, it has to be able to infect the host cells, Cobey says. Avian cells have slightly different surface receptors than human cells, making it more difficult for human viruses to recognize and latch onto the avian cells. Sometimes the human flu viruses adapt, or mutate, to better grab the avian host cell receptors. This can be bad, McMaster University’s Miller says. These adaptations sometimes happen in the hemagglutinin protein, he adds. “This is the antigen that we care about most because that’s the viral protein that our antibodies are directed against. Seasonal flu vaccines are specifically designed to raise antibodies against hemagglutinin.”
Flu viruses that develop mutations in their surface proteins during vaccine manufacture may no longer match the ones circulating in the wild, and therefore the vaccines can’t protect people who receive shots as well. “As a result of that, vaccine effectiveness can suffer, though we can’t easily predict when this will happen,” Miller says.
The two other ways of growing viruses for flu vaccines don’t have this problem because they don’t use eggs to replicate. In recombinant vaccine technology—the most recently developed of the three methods—scientists take antigens from the WHO-supplied viruses, transcribe them into DNA, then use a plasmid to insert the sequences into the cells of Spodoptera frugiperda, commonly called the fall armyworm, says Litjen Tan, chief strategy officer at the Immunization Action Coalition (IAC). The armyworm cells produce large amounts of the flu antigens, which are exact copies of the ones in the viruses from the WHO. Scientists separate and collect the antigen proteins, purify them, and put them in flu vaccines in place of inactivated viruses. The pharmaceutical company Sanofi Pasteur makes this type of vaccine under the name Flublok.
Flucelvax, a cell-based vaccine from Seqirus, is also made without eggs. Scientists grow the virus in a line of mammalian kidney cells called Madin-Darby canine kidney (MDCK), says Ethan Settembre, vice president of research at Seqirus. “The virus infects the cells and then generates more viruses, which float around in the media,” he says. Scientists separate the media containing the virus, then treat the purified virus with a chemical inactivator to kill the viruses, so they’re no longer infectious. The researchers then burst apart the virus to isolate just the outer surface of the cells, which contains the key proteins that are needed for the vaccine. Unlike in the past, the entire process of making Flucelvax is now completely egg-free—even from the original WHO-supplied virus, according to Settembre. Because the virus doesn’t have to adapt to grow inside the mammalian cells, scientists don’t need to be concerned about the viruses evolving.
The egg-based vaccines may be slightly cheaper than egg-free ones. According to the CDC, the adult Flucelvax Quadrivalent (meaning it inoculates against four types of influenza) vaccine costs the public $22.77 per dose, while an equivalent egg-based vaccine from Sanofi is $16.94 per dose. Another egg-based equivalent, the Flulaval Quadrivalent from GlaxoSmithKline, is $15.77 per dose. Data for the Flublok vaccine were not available.
Does it Matter?
When I got sick in 2014, my obstetrician pumped me full of Tamiflu, a drug designed to prevent flu symptoms or to lessen their duration and severity. I isolated myself from my asthmatic toddler, who was also taking Tamiflu. He didn’t get sick, but I spent a lot of anxious nights imagining us both in hospital rooms. My pregnancy turned out OK, and my daughter is fine (although not so great at listening, but I’m not sure I can blame Tamiflu for that).
Some peer-reviewed studies have shown that both egg-free vaccines, Flucelvax and Flublok, have been more effective at preventing illness or inducing an immune response than egg-based vaccines during certain seasons (N. Engl. J. Med. 2017, DOI: 10.1056/NEJMoa1608862; Clin. Infect. Dis. 2018, DOI: 10.1093/cid/ciy097; Proc. Natl. Acad. Sci.U.S.A. 2017, DOI: 10.1073/pnas.1712377114). These studies tend to be small or look at only one age group. The Department of Defense is currently conducting a very large study comparing the effectiveness of egg-based versus non-egg-based vaccines that should give some more concrete answers, according to Tan at IAC. That study is scheduled to be completed in September 2021. There are other studies in process measuring the effectiveness of different vaccines.
For now, scientists and health experts are divided on whether there’s enough evidence to tell people to get one flu shot over another.
“While there can be changes to flu viruses as they’re grown in eggs, many times it doesn’t matter,” says David Greenberg, the head of global medical strategy at Sanofi, which makes Flublok as well as egg-based flu vaccines. A flu vaccine’s effectiveness depends not only on factors like drift but also on a patient’s age, underlying medical condition, and use of medication that causes immune suppression, Greenberg says. “Egg-based vaccines are extremely useful. Even in years where effectiveness of standard egg-based vaccines is in the midrange of 40–50%, this equated to tens of thousands of hospitalizations prevented and thousands of deaths prevented,” he adds.
Tan and Fauci agree. “You shouldn’t downplay or pooh-pooh eggs. It’s worked reasonably well for years,” Fauci says. “I don’t think we want to go out and say that one vaccine is better than the other because, honestly, there is very little data that suggests that the differences between cell- and egg-based vaccines are leading to significant differences between the two vaccines,” Tan says. Some scientists are beginning to look into this, he says, but there’s not enough evidence yet, and he warns against prematurely switching away from something that works. “We want to make sure that we continue to produce our vaccines to the capacity that we need to,” Tan says, adding that it would be challenging to replace the infrastructure that produces flu vaccines in eggs.
The scientists working directly on the research see things a little differently. “I think we now have enough information to know that it would be optimal to get away from producing vaccines in eggs to avoid these egg-based mutations from happening,” McMaster University’s Miller says. “And I think in general the field understands this as well.” The problem is that it can be difficult to get US Food and Drug Administration approval to grow flu in new substrates, he says, but companies are moving “in the direction of trying to get away from our reliance on eggs for vaccine manufacturing.”
New vaccines are likely to be produced without eggs, says Clement Lewin, associate vice president of research and development strategy at Sanofi. A body of evidence is growing that there is a difference in the performance of egg-based and non-egg-based vaccines, he says, and we need to go where the data are.
Given the choice, both Cobey and Miller say they would get a non-egg-based vaccine over an egg-based one. But everyone I interviewed for this story stressed that getting any flu vaccine is better than none. All kinds of flu vaccines decrease the chances of getting sick. “We’re now in an era where there are different kinds of vaccines,” Lewin says. “You need to get a flu vaccine, but you need to figure out which might be best for you as an individual.”