Our Changing Ocean
Beyond the meadows of wildflowers and tree-covered mountains lies the vast blue ocean. Whether it’s in our backyard or a wonder only seen on television and in books, the ocean plays an important role in all of our lives. The ocean covers more than 70% of our planet, regulates Earth’s climate, and produces most of the oxygen we breathe. And we are seeing unprecedented changes in our ocean today.
What’s happening to the chemistry of our oceans?
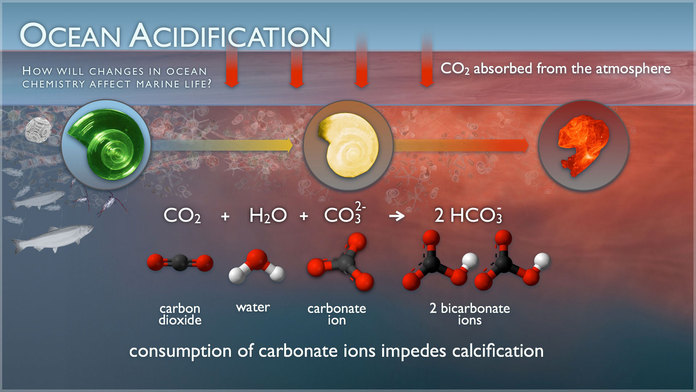
Our actions have a direct effect on ocean chemistry and marine life. About 24% of the carbon dioxide (CO2) pumped into the atmosphere from cars, factories, and power plants is making its way into our ocean, decreasing the pH of seawater in a process called ocean acidification (OA).1 When CO2 dissolves in and reacts with water, carbonic acid is formed and quickly dissociates into bicarbonate and hydrogen ions.2 Hydrogen ions then bond to carbonate ions already in the water to produce bicarbonate:
(1) CO2 (g) + H2O (l) ←→ H+ (aq) + HCO3- (aq)
(2) CO32- (aq) + H+ (aq) ←→ HCO3- (aq)
The NOAA Ocean Acidification Program better prepares society to respond to changing ocean conditions and resources by expanding our understanding of ocean acidification through interdisciplinary partnerships, nationally and internationally.
The NOAA Office of Education advances STEM education by providing scholarships and collaborating with universities to prepare students from diverse backgrounds for careers in NOAA-related fields. The NOAA Office of Education also offers competitive grants and establishes partnerships to integrate NOAA science into schools and informal education organizations.
Since the Industrial Revolution, global seawater has dropped by about 0.1 pH units, which represents a 30% increase in acidity.3 The average pH of seawater is currently around 8.1 and is expected to decrease an additional 0.3 to 0.4 units by 2100 (an additional 100-150% increase in acidity) if CO2 emissions continue in a business-as-usual scenario.3.
OA is a global phenomenon that has impacted and will continue to affect marine organisms and human communities that depend on a healthy ocean. Yet, a public poll in 2010 revealed that only about 23% of the population of the United States is aware of ocean acidification.4 Through the National Oceanic and Atmospheric Administration’s Hollings and Educational Partnership Program Undergraduate Scholarships, students are working with researchers to answer critical questions about ocean chemistry and raise awareness about ocean acidification.
Investigating the intersection of chemistry and marine life
One avenue of research that scientists and students are investigating is the relationship between ocean chemistry and marine life. As equations 1 and 2 show, adding more CO2 to seawater translates into fewer carbonate ions in the water. This makes it more difficult for organisms, such as corals and mollusks, to get the calcium carbonate they need to build their skeletons or shells.
The availability of calcium carbonate for shell building is referred to by researchers as the saturation state (Ω). A low saturation state due to ocean acidification can be thought of as “osteoporosis of the sea” because it causes shell-building organisms to build frail, or malformed, shells as larvae. In fact, it was the massive die-off of larval oysters in the Pacific Northwest of the United States that triggered national interest in ocean acidification in the late 2000s.
An increase in ocean acidity can have a variety of effects beyond weakening shells. For example, it makes it difficult for some organisms, such as juvenile salmon and reef fish, to avoid predators because it alters their sensory input for smell.5
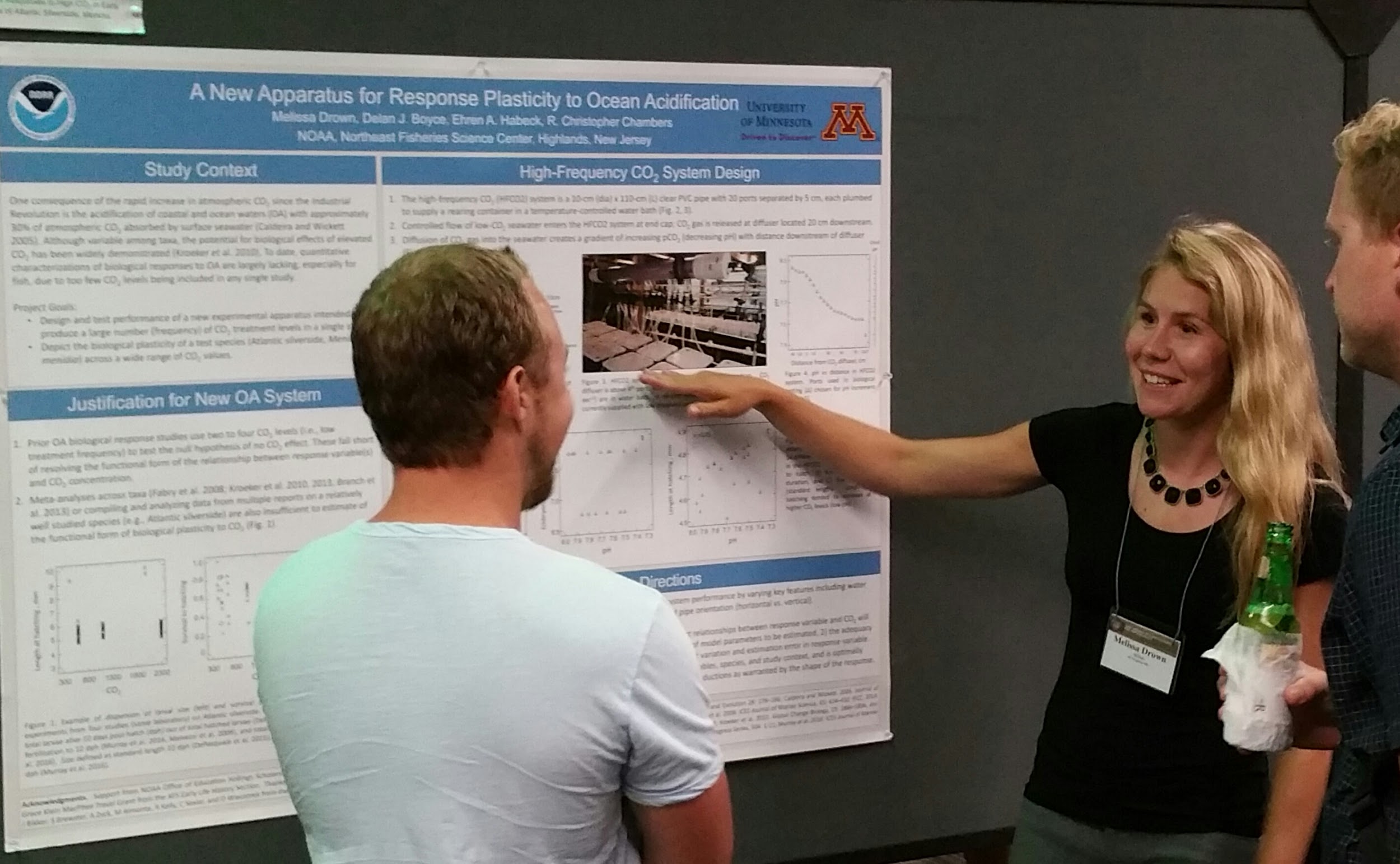
“Ocean acidification has been used in my undergraduate chemistry classes as a real-world example of how important chemistry is and how it can impact marine organisms,” said Melissa Drown, a 2017 Hollings scholar and senior at the University of Minnesota. Drown will be heading to the University of Miami to pursue her doctorate in marine biology and ecology. She worked with Dr. Chris Chambers at the James J. Howard Marine Sciences Laboratory in New Jersey to design a system capable of generating 21 unique and stable CO2 concentrations.
This system allows scientists to identify and pinpoint how ocean acidification impacts marine organisms at many CO2 levels. “Normally research studies look at two or three CO2 levels; this new experimental apparatus will allow research to go beyond answering whether or not marine organisms are impacted by ocean acidification,” said Drown. “It will help us understand thresholds of when an organism is impacted and what this means with projected increases in CO2 concentrations,” she added.
The team, including Hollings scholars Claire Hacker, Sarah Brewster, Amy Zyck, Raven Benko, and Megan Dotterweich, is currently using the experimental apparatus Drown helped design to determine the impacts of ocean acidification on Atlantic silverside, a small foraging fish that is an important food source for commercial fish species. The team will soon transition to look at the American lobster, an important economic species in the northeastern United States.
Top left and right: Hollings Scholars Raven Benko and Megan Dotterweich seining the shores of Sandy Hook Bay for ripe adult Atlantic silverside. Bottom left: Students collect milt from males and eggs from females. Bottom right: A peek through a microscope at Atlantic Silverside eggs attached via egg filaments to lab screening.
The power of communication
One NOAA Hollings Scholarship alumnus went on to pursue doctoral research focused on building additional geochemical tools that can elucidate the effects of ocean acidification in coral reef ecosystems. Isaiah Bolden, a passionate geoscientist and educator pursuing his Ph.D. in chemical oceanography at the University of Washington (UW), was excited to further his research on ocean acidification after gaining experience translating science for decision-makers during his summer 2014 internship with NOAA’s Ocean Acidification Program.

After the internship, Bolden joined Dr. Alex Gagnon’s lab group at UW to investigate how environmental stressors like ocean acidification influences the chemical composition of coral skeletons and lagoon waters. Records generated from this research can help scientists distinguish between natural biogeochemical variability and the effects of increasing anthropogenic impacts in these dynamic environments, often considered the “rainforests” of the ocean due to their biodiversity.
During one of Bolden's most memorable experiences as a graduate student, former U.S. president Barack Obama approached his lab group as they were conducting field research in French Polynesia. As the team explained their research, the former President asked questions that demonstrated a clear understanding of ocean acidification and the consequences for marine life, leading Bolden to affirm that when communicated well, environmental research can leave a meaningful and lasting impression on public officials and leaders.
Read more about Isaiah Bolden in A Peek Inside the Life of a Geochemistry Grad Student
The future of OA research
OA research has come a long way in the past decade, and it is continuing to move forward. Researchers are using more sophisticated technology, including unmanned sampling vehicles and models, to measure and predict changes in ocean chemistry. Additionally, scientists are investigating the impact of multiple stressors on marine organisms and whether certain species will be able to adapt quickly enough to survive in our changing ocean.
How you can help
As you’re focusing on the environment on Earth Day and during Chemists Celebrate Earth Week, remember that you can reduce your carbon footprint, not only for the land but for the sea. Ocean acidification is a global issue, but no act is too small to help. From biking to work to reducing meat and dairy intake, there is a long list of activities that can improve your life, the lives of marine organisms, and the health of the ocean we are connected to wherever we may live.
References
1. Global Carbon Budget 2017. Le Quéré, C; Andrew, R. M.; Friedlingstein, P.; Sitch, S.; Pongratz, J.; Manning, A. C., et al. Earth System Science Data Discussions, 2017. DOI: 10.5194/essdd-2017-123.
2. Dickson, A. G. The carbon dioxide system in seawater: equilibrium chemistry and measurements. In Guide to Best Practices for Ocean Acidification Research and Data Reporting. Riebesell, U.; Fabry, V. J.; Hannson, L.; Gattuso, J.-P., Eds. Publications Office of the European Union: Luxembourg, 2010; pp. 17-40. .https://www.iaea.org/ocean-acidification/act7/Guide%20best%20practices%20low%20res.pdf
3. IPCC. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Stocker, T. F.; Qin, D.; Plattner, G.-K.; Tignor, M.; Allen, S.K.; Boschung, J.; Nauels, A.; Xia,Y.; Bex, V.; Midgley, P. M., Eds. Cambridge University Press: Cambridge, New York, 2013; 1535 pp. DOI:10.1017/CBO9781107415324.
4. Leiserowitz, A.; Smith, N.; Marlon, J. R. Americans’ Knowledge of Climate Change. Yale Project on Climate Change Communication; Yale University: New Haven, CT, 2010..http://environment.yale.edu/climate/files/ClimateChangeKnowledge2010.pdf
5. Ashur, M. M.; Johnston, N. K.; Dixson, D. L. Impacts of ocean acidification on sensory function in marine organisms. Integrative and Comparative Biology 2017, 57(1), 63-80.
Last updated 4/26/18