Shining a Light on Candles

Glancing up at a 700-person crowd in December 1860, Michael Faraday, the renowned scientist who uncovered the fundamental principles of electrochemistry and electromagnetism, ignited the large room by boasting his best example of nature’s beauty: the humble candle.
Although Faraday’s work led directly to such advances as electric motors, transformers, and generators, he used candlelight to illuminate many concepts in chemistry. “There is no better, there is no more open door by which you can enter into the study of science than by considering the physical phenomena of a candle,” Faraday said at the start of his final Chemical History of a Candle lecture series. “I trust, therefore, I shall not disappoint you in choosing this for my subject rather than any newer topic, which could not be better, were it even so good.”
Historical records show that the ancient Egyptians used an early form of candles 5,000 years ago, and many civilizations invented and advanced them independently. People have burned candles with animal fat, wax from whale heads, and beeswax, and the Romans are credited with adding a papyrus wick to the equation over 2,000 years ago. Three ingredients—fat, fiber, and fire—describe a basic candle simply. But to really understand the science at play, we have to consider the microscopic level of how each component contributes to the light and heat we get from burning candles.
What makes a flame
For millennia, humans have used a flint, match, or lighter to heat solid waxy carbon chains embedded in the candle’s wick, melting them and then vaporizing them, providing enough activation energy to spark the exothermic reaction.
Yet it wasn’t until the late 18th century that scientists were able to elucidate the science behind the flame. Previously, they had attributed combustion to a special substance called phlogiston, which they believed to be intrinsic to any matter capable of burning; they had not conceived of individual substances. But with a series of experiments in 1772, French scientist Antoine Lavoisier proved that phosphorus and sulfur gain weight after burning. This convinced chemists that combustion is a reaction between a material and a component of air, which Lavoisier named oxygène.
Lavoisier also coined the term oxidation to describe oxygen’s role during combustion. Since the oxygen reacts by snatching other atoms’ electrons, the term came to refer more generally to any reaction in which electrons are lost. Unlike a rusty metal fence oxidizing bit by bit, a burning flame reacts fast and with enough energy to sustain itself. For organic molecules like wax, burning—or combusting—corresponds to the molecule breaking down with heat and oxygen. Shattering each carbon bond releases energy that was previously tied up in the molecule, and new bonds form with oxygen to produce carbon dioxide and water, which are stabler configurations of the atoms. Stable chemical bonds have less potential energy; therefore, they are stronger and release lots of energy when they form. That energy helps propagate the reaction to activate more bond breaking. So long as enough oxygen and fuel exist nearby, the wick's wax keeps burning.
What’s actually burning
It may come as a surprise, but the solid wax isn’t what’s burning. In fact, the solid wax blanketing the wick on a new candle is exactly what makes it slower to light the first time—you have to melt away that wax coating. And anyone who has drowned the flame in a pool of melted wax could guess that it’s not the liquid form that is burning either.
Heat from the flame melts the nearby wax, which is then drawn up into the wick via capillary action. When the liquid wax gets to the flame, it evaporates, and that gaseous form is what actually burns.
This necessary physical transformation also tells us why some waxes burn faster or slower than others. “Certainly the burning rate and the types and amounts of soot that are formed definitely are impacted by the type of wax,” says Brandon Rotavera, a physical chemist who teaches combustion chemistry courses at the University of Georgia. Candle waxes are all hydrocarbons, but you can glean a lot about wax behavior from their chemical formulas. A long carbon chain with double bonds and carbon rings will burn at lower temperatures and emit more soot than a saturated hydrocarbon unencumbered by side chains, Rotavera says. These unsaturated hydrocarbons have higher carbon-hydrogen ratios, making them harder to burn.
He adds that the chemical nuances of burning show up in his lab’s research on how sustainable biofuels combust in existing gasoline engines and ones designed for more-sustainable fuels. Blending in some biofuel molecules affects when fuel ignites. And Rotavera says adding only about 5–10% biofuel content dramatically changes the combustion efficiency. Since many viable biofuels exist, it’s important to study how each individual one behaves. “We really look at a molecule—and we replace a carbonyl group with an ether group or a hydroxyl group,” he says. “And that completely changes the type of reactions that unfold.”
The same connection between molecular structure and macroscopic behavior plays out with candles. Plant-derived soy or palm waxes can burn slowly or quickly depending on their composition. Stearic acid is a carboxylic acid and a primary component in soy wax, with 18 carbons and 2 oxygens. Those 2 oxygens on each stearate molecule introduce attractive intermolecular forces that stabilize the wax’s liquid and solid forms—making it harder to vaporize. Since plant-derived waxes are less likely to contain impurities such as benzene, many regard them as cleaner burning than petroleum waxes.
Paraffin wax, the most common candle fuel, is a mixture of straight-chain hydrocarbons at least 20 carbons in length. Since it’s a petroleum by-product, it’s less expensive than alternatives such as beeswax, but it tends to burn faster than other waxes, making it better for emitting fragrance. Candlemakers can raise paraffin wax’s melting point by adding stearic acid to the mixture for a less expensive alternative to beeswax.
Solid wax undergoes physical and chemical transformations whenever a candle burns. Since each transition depends on wax properties such as carbon chain length and molecular structure, candles underscore how important it is to choose the right fuel.
Designing the best wick
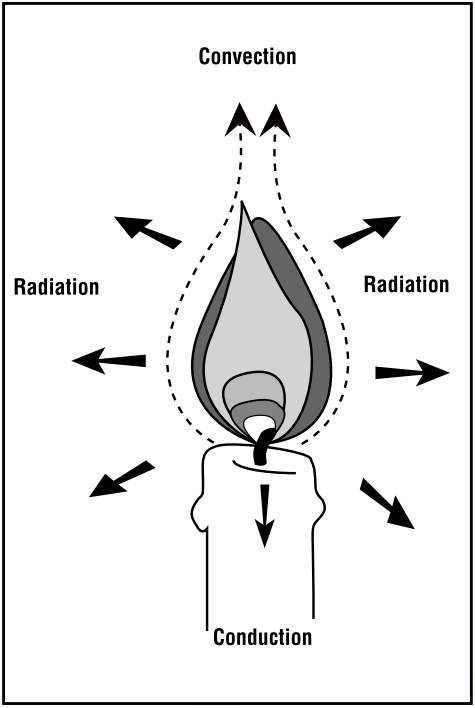
Wicks submerged in that complicated wax are more than just string; they help regulate how quickly the wax evaporates and burns. Only the wax high in the wick gets hot enough to vaporize and ignite, but the wick also conducts heat downward, melting solid wax into a liquid reservoir to replenish the wick. After gaseous wax molecules burn off as blue flames, more wax molecules replace them, flowing up the wick through capillary action like a conveyor belt.
Soaking the wick in a fire-retardant chemical keeps it from burning up so fast that it drowns in molten wax. This treatment, called mordanting, involves chemicals such as borax (sodium borate) that bind to a wick’s cellulose fibers and help form a protective layer of char. Wicks must typically also be braided tightly to control fuel burning. A loosely braided wick draws up fuel well but leaves more surface area exposed to oxygen. “You've got more of the fuel exposed to the burning environment,” Rotavera says. “They've got more localized hot spots, more localized points of ignition.”
And in slow-burning beeswax candles, unburned wax, pigments, and fragrances can clog up the capillary flow in thin wicks. More-robust square wicks are designed to prevent that clogging by absorbing melted beeswax better. For all wicks, the mission is the same: soak up fuel just fast enough to burn slowly and evenly.
Convection and color
Regardless of what wax or wick a candle contains, they all look quite similar when burning: a multicolored flame in a distinct teardrop shape. That characteristic shape arises from convection. Since the highest temperature of the candle occurs near the bottom of the flame, this area has the least dense air. To fill the oxygen density gradient, the flame draws in cooler, denser air from below, creating an upward current that contours the teardrop flame.
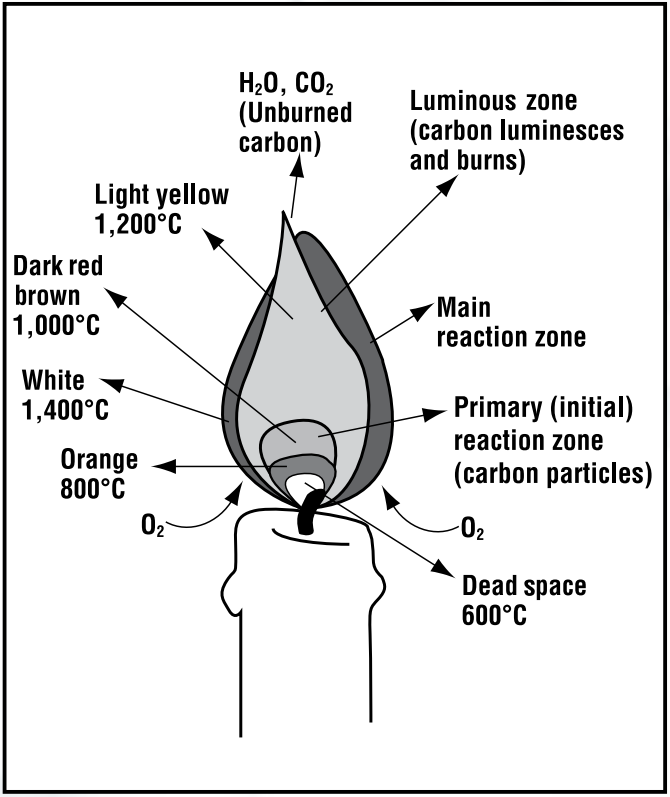
The temperature gradient in a candle’s flame also explains its colors. Of the flame’s three distinct regions, the first and hottest is the blue flame—a region where evaporated wax combusts completely. Complete combustion means the total oxidation of the hydrocarbon into water and carbon dioxide, with no side reactions or side products. After heat snaps carbon bonds, C–H radicals get excited and emit blue light, Rotavera says. In contrast, hydrogen gas burns with a colorless flame.
The grayish region circling the top of the wick above the blue experiences incomplete combustion. The energy in this coolest region is still high, but there isn’t enough oxygen around for the evaporated wax to burn fully. Partially fragmented bits of the carbon chain recombine into molecular rings like benzene, and those rings multiply around each other. “Multiring systems grow and grow and grow until they get large,” Rotavera says. “And those aggregate into a particle.” These dark, carbon-rich particles called soot exit the flame without burning. The flame’s heat then makes the soot glow bright yellow in a physical process called incandescence.
“You would hardly think that all those substances which fly about London, in the form of soots and blacks, are the very beauty and life of the flame,” Faraday said in his lectures.
An experiment on the International Space Station proved that in space, flames don’t undergo the same convection as they do on Earth, because low-density air doesn’t rise without gravity. This lack of buoyant convection creates a spherical flame. In microgravity, spherical flames are bluer and less yellow. Scientists believe that without convection, enough heat stays well distributed around the burning molecules, which would otherwise leave as unburned soot. This stagnant blanket of heat leads to more complete combustion and less incandescence—a dimmer but a more efficient flame.
Learning from candles
Deeply understanding any chemical technology—even deceptively simple ones like candles—allows us to improve on it. Faraday, for example, couldn’t help but point out one glaring issue with his contemporary candles. “You have, I know, often smelt the vapor of a blown-out candle—and a very bad smell it is,” he said. Today, on the other hand, many people purchase candles specifically for their scents.
Studying candle flames might help us improve technology beyond just candles. NASA’s microgravity experiments, for example, are helping engineers discover how to design engines that burn with less soot. They also learned that diffusive flames like candles can keep burning at low temperatures in microgravity, and that discovery may inspire new, cleaner combustion engines.
“When I think about the candle, I think about these sporadic kinds of moments where there's a flickering of the flame or popping noise or something like that,” Rotavera says. Similar instabilities happen unpredictably in even the most cutting-edge engines. As the scientific community focuses on making the most-sustainable engines, weeding out inefficiencies is critical. “That really is something important to predict because engines need to operate routinely and consistently.”
“We come here to be philosophers,” Faraday said. “I hope you will always remember that whenever a result happens, especially if it be new, you should say, ‘What is the cause? Why does it occur?’ and you will in the course of time find out the reason.”