Going Skin Deep: The Culture and Chemistry of Tattoos
By Frankie Wood-Black
What do Ötzi the Iceman, Tofi women of New Guinea, and Kat Von D have in common? Believe it or not, the answer is tattoos! Tattooing has been a part of human history for thousands of years, and for a variety of reasons. Ötzi, the 5300-year-old, health-riddled, mummified hunter discovered preserved in a glacier in the Ötztal Alps, has 61 tattoos located near his joints, suggesting they may have been associated with an ancient treatment for arthritis. Tofi women have swirl patterns on their faces, indicating their family lineage. And Kat Von D, a popular American tattoo artist and reality TV star, holds the Guinness World Record for most tattoos given to a single person in 24 hours: a whopping 400!
Whether an expression of religious beliefs, rites of passage, or personal interests, tattoos are here to stay. But have you ever really thought about the chemistry of tattoos? What exactly is in those inks, and how safe are they?
Getting ink into the skin
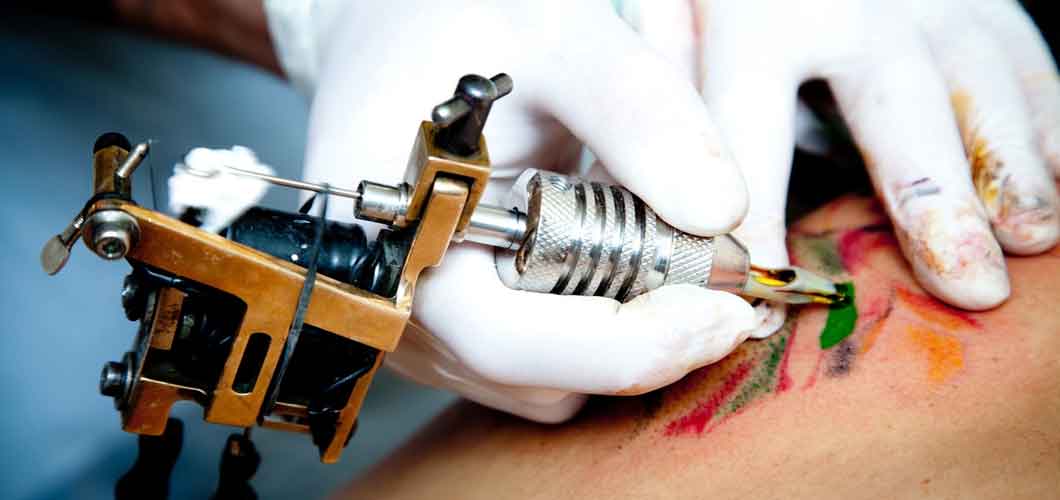
Permanent tattoos are made by injecting an ink into the skin using needles. When a tattoo needle punctures your skin, it causes a tiny wound. Your body responds to all wounds by sending macrophages to close the wound and swallow up any foreign invaders. In the case of tattoo ink, the pigment particles are too large for the macrophages to destroy, so they get stuck in the dermis.
A tattoo will fade if your immune system ever succeeds in breaking up the pigment particles. If you want to remove a tattoo, you can get a laser treatment to target a single color in your tattoo and break up the pigment particles into something the macrophages can handle.
Reactions Science Video Series
If you don’t have a tattoo, you probably at least know someone who does — but what’s the chemistry behind tattoos? In this Reactions video we explore what tattoo ink is made of, why this body art is permanent (whether you like it or not) and other cool facts.
What’s in tattoo inks?
Tattoo inks are solutions comprised of a carrier and a colorant. The carrier is the fluid that is used to transport the colorant to the application location. It may contain glycerin, water, isopropyl alcohol, and witch hazel.
Tattoo colorants are typically pigments — intensely colored compounds that can reflect light in the visible region of the light spectrum — as opposed to dyes, which require a physical or chemical interaction to be anchored into place. In other words, dyes must react with the surface of the skin to develop their color and stay in place. Conversely, pigments provide color without needing a chemical reaction, and are held in place by intermolecular or physical forces.
Historically, pigments used in tattoo inks derived from mineral or geological sources to produce certain colors and hues. For example, carbon (carbon black) and iron oxide were used to produce a black ink. Cinnabar, a mercury sulfide compound, was used to produce red hues. Cadmium compounds, such as “cadmium red (CdSe)” or “cadmium yellow (CdS or CdZnS),” were used to produce shades of red, orange, and yellow.
For the last 20 years, ink manufacturers have moved away from primarily mineral-based pigments to organic ones. Over 80% of the colorants used today are carbon-based, and approximately 60% of these organic pigments are azo pigments. About 30% of the pigments and dyes are approved for cosmetic use, while a number of others were originally developed for industrial applications, like paints or textiles.
Tattoo inks also include a number of additives, such as surfactants, binding agents, fillers, and preservatives. Many of these additives are employed to keep the pigments in a uniform suspension to avoid microorganism growth in the product after opening.
Potential risks
There are very real risks involved with inks and the tattoo process. The most common of these risks is that of an infection. Other known adverse reactions include allergic-hypersensitivity and auto-immune reactions, granulomas, and interferences with medical diagnoses and treatment.
Also concerning, there are more than 200 colorants and additives in current use to produce tattoo inks, but their long-term outcome in the body is not well understood. This is especially true for industrial pigments, which are not tested for cosmetic use. Azo pigments are known to release carcinogenic aromatic amines as they break down, specifically when exposed to solar and ultraviolet radiation.
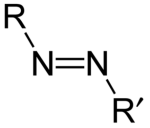
Azo Compound
IUPAC defines azo compounds as derivatives of diazene (diimide), HN=NH, wherein both hydrogens are substituted with hydrocarbyl groups.
Government regulations for tattooing are varied and tend to be associated with location, licensing of the tattoo parlor, and consent for minors (see regulations by state). The primary risks monitored under these regulations are the possibility of infection and the transmission of disease through unsanitary procedures or conditions.
Long-term risks are associated with the quantity and type of pigment left in the skin producing the tattoo. An investigation by the Joint Research Centre (JRC) determined that inks may contain up to 60% by weight of the pigment component. But, what does that equate to under the skin? The research showed that, on average, a tattoo contains 2.53 mg of colorant per square centimeter, so a 400 cm2 (approximately 6"x10") will contain 1 gram of pigment. Thus, a little bit of pigment goes a long way, and not all pigments have potentially hazardous ingredients — which means the individual risk associated with a tattoo is highly variable.
Potential Degradation Pathways for Azo Pigments
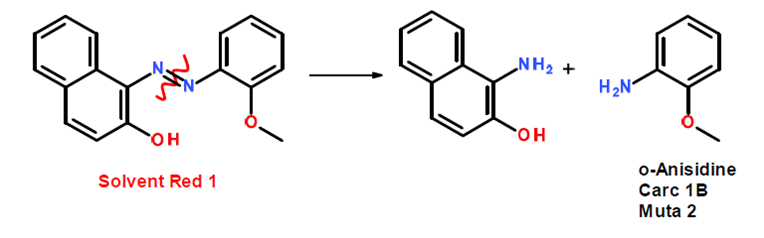
Azo compounds may undergo reactions that cause the compound to cleave into a aromatic amines. Some degradation products, such as aniline, anisidine, and toluidine, have been identified as potential carcinogens.
For example, solvent Red 1 can cleave at the N=N bond:
Information is important to minimizing any risk. While there is no federal standard, distributors of quality inks now provide lists of ingredients used in their products as well as information about the conditions of use and other warnings. At a minimum, the packaging should reference the name and address of the manufacturer, an expiration date, conditions of use and warnings, batch identification, list of ingredients, and a guarantee of sterility. The ink should also be packaged for single use.
In spite of the risks, tattoos are a part of world culture and its long history. We humans seem to like to add a bit of color and self-expression to our bodies and surroundings. And, it seems the chemical industry is doing quite a bit of investigative research that will make tattooing even more intriguing in the future.
This article is adapted from The Chemistry of Tattoo Ink by Christine Dunne, published on the ACS Undergrad Blog.
References
What’s That Stuff? Tattoo Ink. Chemical & Engineering News. 2009.
“Chemistry under your skin? Experiments with Tattoo Inks for Secondary Students.” M. Stuckey and I. Eiks. Journal of Chem. Ed. 2015, 92, 192-134.
“Historic Mineral Pigments: Colorful Benchmarks of Ancient Civilizations.” Mary Virgina Orna.
Safety of tattoos and permanent make-up: Final report. Paola Piccinini, Sazan Pakalin, Laura Contor, Ivana Bianchi, Chiara Senaldi; EUR 27947 EN; doi: 10.2788/011817