The Great Thanksgiving Chemistry Debates

Thanksgiving is a time to spend with cherished loved ones, eating time-honored foods, and reflecting on what we are grateful for. It’s also a time of great debates. Will the joyous feast be at Grandma’s or Uncle Fred’s? Do you make roasted sweet potatoes or mashed Yukon golds? Do you serve ham, lamb, or roast beef alongside the main attraction—turkey? And if you’re a football fanatic, do you root for the Chargers or Cowboys?
The answer to many of these debates—the food ones, that is—can be found in chemistry. Read on to find out how chemistry plays into your great Thanksgiving debates.
White meat vs. Dark meat
When you get to the table, invariably you’re going to have an inner debate with yourself about choosing white meat or dark meat. Some people go for white meat believing it’s a healthier alternative to the more fatty dark meat. But did you know that dark meat has more myoglobin than the white meat? This is important to note because myoglobin plays a role in carrying oxygen to muscle tissues. The amount of myoglobin is dependent on the muscle type. Slow twitch fibers work more slowly for a longer time, as when the leg of a turkey supports its body. These fibers require more oxygen to function and thus have more myoglobin.
Fat is also a key energy source, which is why dark meat tends to be slightly higher in fat. Fast twitch fibers are found in white meat. They work fast but tire quickly, as when a turkey uses its breast muscles for short bouts of flight. Because these fibers don’t use much oxygen, they require less myoglobin. They also get more of their energy from glycogen and tend to be leaner.
Tryptophan vs. Carbohydrates
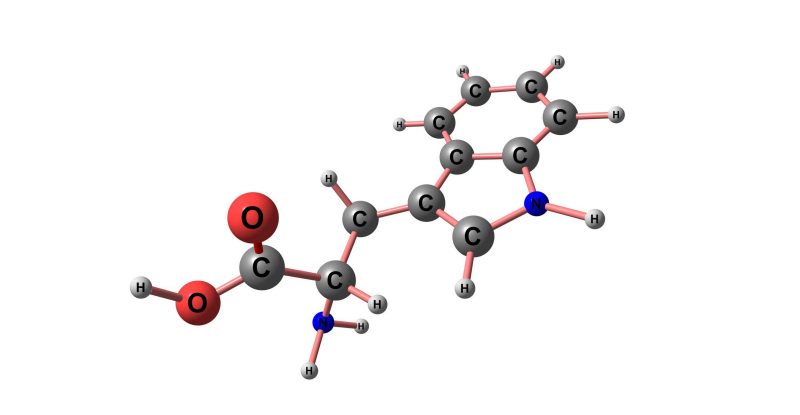
Inevitably, after the Thanksgiving feast, you get sleepy. You can blame the compound tryptophan for that. Tryptophan, C11H12N2O, is an amino acid that contains an α-amino group, an α-carboxylic acid group, and a side chain indole. It can be found in eggs, salmon, elk, dairy products, seaweed, soy sauce, sesame seeds, cashews, and, of course, turkey. Although turkey is generally thought of as being high in tryptophan, cheddar cheese contains more tryptophan on a gram for gram basis.
Tryptophan has been shown to support the body’s synthesis of serotonin and melatonin which helps you sleep. But it also helps to support the immune system, circulation, the central nervous system, enzyme production, and can be converted into niacin (Vitamin B3).
While tryptophan typically gets blamed for the post-Thanksgiving nap, the sleepiness is more likely due to all of those carbohydrates on the menu. Carbohydrates trigger the pancreas to release insulin to regulate the amount of glucose in the bloodstream. The high intake of carbohydrates or sugars results in a higher dose of insulin, which, in turn, quickly drops the blood sugar level, reducing the available energy. And that makes you feel tired. Additionally, humans have a normal biological tendency toward sleep right after eating.
Light browning vs. Extra browning
Whether your turkey, ham, sweet potatoes, rolls, and pies are a light golden brown or a deep caramel color, browning is all about the Maillard reaction. The Maillard reaction is an umbrella term for a group of reactions responsible for a number of flavors at the Thanksgiving table.
The reaction occurs around 140 – 160 °C (280 – 330 °F). It starts when the carbonyl group in a sucrose molecule reacts with an amino group in a protein to make a glycosylamine compound. The glycoslyamine isomerizes, then breaks down into a variety of compounds that produce those brown colors and roasted, nutty aromas that make our mouths water.
As the heat increases, other reactions, such as caramelization and pyrolysis, can occur, producing additional flavors and textures. During caramelization, the heating of the sugars produces caramelans (C24H36O18), carmelens (C36H50O25), and carmelins (C125H188O80). The higher heat causes the sugars to decompose and condense, evolving water and other volatile compounds to produce the nutty flavor, color, and texture that enhances those yummy candied yams.
The Maillard and caramelization reactions are why glazing your ham with honey produces that extra-special crust and why an egg-wash makes your rolls a golden brown. To get the color and flavor you want, adjust baking times and temperatures for your desired perfect glaze.
The chemistry behind the "browning reaction" is responsible for those good smelling steaks. Delve into how this process works to learn the science behind the deliciousness of your cooking.
Thick gravy vs. Thin gravy
The thickening of gravy (and other liquids, like cranberry sauce and fruit pie fillings) depends on starch reactions. This introduces another debate: cornstarch, flour, or arrowroot for thickening?
In all of these starch sources, the starch is comprised of amylose (C6H10O5) and amylopectin (C6H10O5). It is the structure that is important. Amylose is more straight-chained, while the amylopectin has more branches. In preparation of the dish, the starch is brought into contact with a liquid such as the pan juices for the gravy, or the fruit juice for the pie.
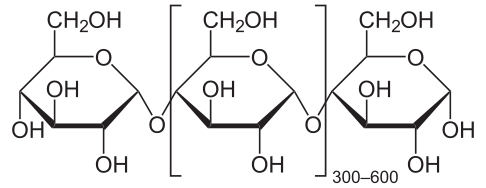
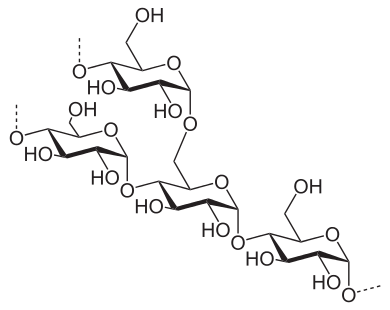
When you first mix your thickener with liquid (be it stock, drippings, or fruit juice), the granules begin to slightly absorb some of the liquid. But there really isn’t much chemistry happening at this stage. You are just trying to get the granules evenly dispersed throughout the mixture, otherwise, lumpy gravy is likely to be on the menu. Once the mixture is heated, the starch begins to gelatinize. The intermolecular bonds break in the presence of water and the increase in energy (from the heat). As the starch molecules are freed up, more hydrogen bonding occurs, forming a network that ultimately thickens or gels.
Whether the starch thickens or gels depends on the ratio of the amylose versus amylopectin, which varies by plant species. Amylopectin forms more hydrogen bonds (and forms them more quickly) than amylose and thus tends to form gels. In both wheat starch (i.e., flour) and cornstarch, the amylose:amylopection ratio is 25:75. So, they thicken at the same rates. However, flour as a lot of stuff in it other than starch (i.e., proteins, other carbohydrates), so it provides a cloudy finish familiar to gravy. Cornstarch is pure starch, so it tends to be more translucent, which is ideal for fruit pies. In arrowroot starch, the ratio can be as high at 1:99, and so are great for anything you want to gel, like fruit pies or jellies.
The Verdict
The Thanksgiving table is filled with not only good food, but good chemistry. One only has to take a whiff to detect vanillin (vanilla) and eugenol (clove). Or look eye the table for emulsions (salad dressings) and suspensions (whipped cream). Or get a mouthfeel of textures created from combining milk and potatoes, melting cheese, and baking of breads. And, ultimately, taste the scrumptious result of reactions and complex chemicals that make us look forward to the Thanksgiving holiday year after year. And, after the meal? There are still choices to be made—what game to watch or when to go shopping!