The Boom in Fireworks
For many chemistry aficionados, explosions are the most fun reactions, with color changes coming in a close second. And on the Fourth of July (Independence Day), explosion enthusiasts get to enjoy some exciting chemistry in action.
Independence Day celebrates the signing of the U.S. Declaration of Independence from England on July 4, 1776. The night before, one of the signers, John Adams, wrote to his wife that the day “ought to be solemnized with Pomp and Parade…Bells, Bonfires, and Illuminations…from one End of this Continent to the other, from this Time forward forevermore.” In the vernacular of the day, “illuminations” meant fireworks or pyrotechnics. Accordingly, fireworks celebrations were first held the next year on July 4, 1777, in Philadelphia and Boston. Thus began an American tradition.
Putting the “pyro” in “pyrotechnics”
Fireworks were invented in China in the 9th century, but the word pyrotechnic (“pyro” meaning fire and “technic” meaning made by art) did not appear in the English language until sometime around 1700. The first fireworks were likely firecrackers, and the first firecrackers are believed to have been pieces of roasted bamboo that would pop or bang when steam in the natural voids—or pockets—would build up enough pressure to burst through the plant walls.
To make an air display, the Chinese introduced gunpowder, creating bamboo mortar shells that were fired into the air. The original formula still dominates the fireworks industry today. Traditional gunpowders are a mixture (by weight) of 15% charcoal, 15% sulfur, and 75% saltpeter (potassium nitrate). When ignited, the gunpowder undergoes the following reaction:
10KNO3 (s) + 8C (s) + 3S (s) → 2K2CO3 (s) + 3K2SO4 (s) + 6CO2 (g) +5N2 (g)
Each kilogram of gunpowder rapidly generates 430 g of gases and 3 MJ of energy, which is released as heat and light.
Putting the fire in the sky
An exothermic reaction is not enough to generate the heavens-bound explosion that Western explorers and tradesmen brought from China to Italy during the Renaissance. As anyone who has ever made a film canister rocket knows, good rocket design is key. As the amount of gas in a sealed container increases, so does the pressure in the container (increasing the temperature increases the pressure even more). If the pressure overcomes the container in all directions equally, you’ll get an explosion. If it comes out of a single point in the container, such as an outlet, it will provide enough thrust to launch the rest of the container into the air.
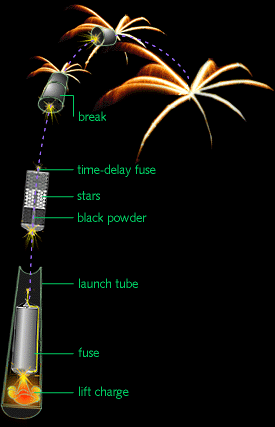
Basic fireworks are simple: They consist of a cardboard shell, which holds the burst component, and a lifting charge to propel the burst component into the air. The lifting charge is ignited via a fuse. A second, time-delayed fuse is ignited with the lifting charge. Several seconds after takeoff, the second fuse ignites the burst charge, which explodes its fiery payload all over the night sky.
Missiles and rockets have a similar design. Although the most common propellant in fireworks is good, old-fashioned gunpowder, composite propellants that combine oxidizers with fuels are more common in the aerospace industry. Oxidizers like ammonium perchlorate or ammonium nitrate are combined with energetic materials like hydroxyl-terminated polybutadiene, polybutadiene acrylonitrile, or trimethylenetrinitramine for the stronger thrust needed to launch into orbit.
Lighting up the night
The burst component contains a burst charge and “stars,” which are individual pellets comprised of:
- Fuel, such as elemental sulfur, carbon, magnesium, or aluminum
- Oxidizers, such as nitrates or perchlorate salts
- Color producers (usually metals or metal salts)
- Binders, such as red gum (acaroid resin), to hold the pellets together
Fuels can be anything that is stable at ambient conditions but that burns hot when ignited, such as elemental sulfur or carbon. Combustible metals, like magnesium, or blends, like thermite, may also be used for extra brightness.
Especially important are oxidizers. Although fireworks are not airtight, there is no way that oxygen from the air can get to the fuel fast enough to support an explosion. So, compounds that can donate a lot of oxygen quickly are necessary. Nitrates and perchlorates are common. Oxidizers can also pull double duty if color-producing metals are the cations in the salts (see below).
So what gives fireworks their color? Metals or metal salts. If you have ever done a flame test, you know that the color comes from incandescence—light produced from the heat of the explosion. The increased energy causes the electrons in an atom to enter an excited state, and when these electrons move from a higher energy state to a lower one, a photon is released. If the energy of the photon is within the visible range of the electromagnetic spectrum, a color is observed and is characteristic of the element being used.
Most commonly used compounds
Strontium or lithium salts | Red |
Calcium salts | Orange |
Sodium salts | Yellow |
Barium compounds | Green |
Copper compounds | Blue |
Combination of blue and red compounds or else cesium, potassium, or rubidium compounds | Purple |
Aluminum, magnesium, or titanium metals | White |
The positioning of the individual stars within the shell of the firework creates the familiar shapes of fireworks such as the peony, chrysanthemum, willow, and crossette. Recent advances in the art have resulted in displays tailored to make smiley faces or hearts.
Starting with a big bang
Sound is a really important part of a fireworks display. Sure, the visual display is amazing, but the bangs, crackles, and whistles contribute to the energy and excitement. Not only do we see fireworks, we feel them.
To get a really good bang, many commercial fireworks add compounds to the shell that produce a larger, louder explosion. These include a potassium chlorate, which is an additional oxidizer, sulfur or antimony trisulfide, and aluminum pieces or other metals.
For a crackling sound, a different “flash and sound” mixture may be added. Originally lead tetroxide was used, but because of environmental concerns, today’s fireworks are more likely to contain granules of bismuth trioxide or bismuth subcarbonate mixed with a magnalium, an alloy of magnesium and aluminum. The rapid combustion of the granules produces the crackling sound.
The whistle is a bit more complex because it occurs from a combination of the combustion of the compounds used and the construction of the tubes. The rapid combustion of the compounds produces standing waves within the tubes which, ultimately, causes the whistle. Various compounds such as gallic acid, salicylic acid, and benzoic acid salts are used.
Playing with fire… safely
Fireworks are explosive and contain a variety of metal salts, oxidizers, and shock-sensitive materials, and, therefore, come with inherent dangers. Individuals staging public displays have to be appropriately licensed and maintain safety.
Families or individuals purchasing fireworks for their own celebrations also need to consider safety. Each year the National Safety Council publishes statistics related to fireworks and safety tips. It is essential that the safety guidelines and instructions be followed. Even so, accidents can still occur, so people have to be prepared.
Explosions and fire aren’t the only concerns with fireworks. Many of the metal salts and oxidizers used have significant environmental impacts. Many manufacturers are trying to find alternatives for some of the toxic metals used in the past. For example, lead tetroxide replaced bismuth compounds that were used to produce the crackling sound effect. Many environmental chemists have also been studying the air concentrations of metals on the fifth of July to gauge the potential health risks associated with firework displays as well as other impacts.
With an estimated $800+ million dollars reportedly spent by Americans on fireworks in 2016, chemists and pyrotechnic artists are always trying to find new compounds to produce different colors and new packing arrangements to produce different shapes. Scientists are also looking for new combinations to get just the right “flash, bang” while reducing toxicity and minimizing the environmental impact.
Of course, fireworks aren’t just for the Fourth of July. We see them at sporting events and other celebrations. So, go out and enjoy the displays. Marvel at the colors. Marvel at the designs. And, know that—somewhere—a chemist is out there right now working on making fireworks even better.