Nanoscientists Taking on Climate Change
The climate crisis raises countless research questions. How can we use solar energy when the sun goes down? How can we produce cell phones and other products without generating greenhouse gases? How can we recapture carbon we’ve already released?
Increasingly, chemists turn to nanoscience to answer these questions. At the nanometer scale, materials take on optical, chemical, physical, and electrical properties that differ from the same material in larger form. The surface-to-mass ratio for nanoparticles is huge, which is a major boon for catalysis. Nanoparticles are often smaller than visible light wavelengths (~400 - 700 nm), making them invisible to the eye. At the same time, the small size means the quantized energy levels dominate the electronic properties, making their behavior dependent on particle size and shape, as well as composition. Nanoscientists can harness these unique properties to combat climate change in many ways.
Consider, for example, the work in quantum dots that recently was awarded the Nobel Prize. These uniformly sized and ordered nanoparticles have paved the way for more efficient screens, lasers, and photovoltaic cells.
“I call it nanoscale solutions to giga ton problems,” says Vinayak Dravid, a professor of materials science and engineering at Northwestern University in Illinois, who uses nanoparticles to turn ordinary household sponges into tools for recapturing and reusing spilled oil.
Governments and international organizations are encouraging this work. For example, the National Nanotechnology Initiative’s current National Nanotechnology Challenge on climate change, Nano4EARTH, challenges nanoscientists to address the warming planet in many ways, from finding new approaches to reducing greenhouse gas emissions to building resilience to the climate changes that are already underway.
Nanoscientists across the globe are answering this challenge within a wide range of disciplines—from marine biology to chemical manufacturing. We talked to some of these scientists about how they tackle climate challenges with big innovations using the tiniest tools.
Celebrate National Nanotechnology Day
October 9 is National Nanotechnology Day. Check out these fun ideas to investigate advances in nano science and how they impact our everyday lives.
Nano comes into focus
Dravid uses nanoscience to find better ways to clean up oil spills. “Every day, there are oil spills occurring throughout the world. This is a very pernicious problem,” he says.
He and his team developed nanostructures made of iron and carbon that they spray in a thin layer onto kitchen sponges or foam packaging. The nanostructure coating causes the sponge to absorb oil, rather than water, when placed in oil-contaminated seawater or any oil–water mixture. When the sponge is squeezed, the oil drips out, and the sponge can be used again and again. “The biggest advantage is sustainability—the ability to reuse this [sponge] repeatedly,” says Dravid. Recapturing spilled oil also means lessening the demand for more oil.

Dravid founded a start-up, called MFNS Tech, to scale up this tool and expand it to capture other pollutants, like phosphate, which could then be used as fertilizer.
Dravid came to nanoscience by way of his lifelong love of science. He grew up in India, where his mother offered him a constant supply of books that spurred this passion. Early in graduate school, he became fascinated with materials science. “A lot of the challenges we face are because of materials, and the solutions, I feel, are also in materials,” says Dravid.
In the 1990s, as a young materials science and engineering professor at Northwestern, Dravid saw his first nanoparticle. His colleague and nanoscience pioneer Chad Mirkin was just beginning to develop methods for creating nanoparticles. Dravid, skilled in microscopy, lent a hand by using transmission electron microscopy (TEM) and other tools to visualize the particles that Mirkin created. “At that scale, nothing was explored up to that point,” recalls Dravid. Working late nights and weekends with sometimes temperamental equipment, Mirkin and Dravid refined the tools to create nanostructures, the microscopy power to see them, and the computing power to simulate their properties. Dravid has been hooked on nanoscience ever since.
For students interested in dreaming up new nanoscience-based tools, there’s no one right educational track, says Dravid. “Nano is so vast and so broad. You don’t have to major within nano.” In fact, many universities don’t offer a nanotechnology major at the undergraduate level. Instead, Dravid recommends you consider specializing in nanotechnology within another major.
If you are considering graduate school, nanotechnology programs are quite common. You can also specialize in nanoscience within a graduate program in iInorganic chemistry, polymer chemistry, materials chemistry, and some areas of analytical chemistry.
Nanotechnology to the rescue

Liza Roger, an assistant professor at Arizona State University in Tempe, only recently discovered the power of nanoscience. She grew up exploring rock pools at low tide in her hometown of Normandy, France. The critters she discovered inspired her to become a marine scientist.
Her research interests turned nanoscale when she became a postdoc in the lab of nanoscientist Nastassja Lewinski at Virginia Commonwealth University. There, Roger began exploring whether nanoparticles could protect corals from high temperatures. “Corals are very sensitive to environmental change,” explains Roger. Tiny algae live within coral cells and provide these animals with energy harnessed through photosynthesis. When ocean temperatures rise too high, this symbiotic relationship breaks down, and the corals quickly die.
This disruption is caused, in part, by a heat-induced increase in oxidative stress levels in both the coral and the algae. One way that humans counter excessive oxidative stress is by consuming foods high in antioxidants, like carrots and green tea, explains Roger. Because some nanoparticles have antioxidant capacities, too, she wondered whether she could use them to make the algae more resilient. Treating the partner’s oxidative stress level could help reduce the coral’s stress to a manageable level, says Roger.
She and her team added metal oxide nanoparticles to dishes filled with a species of algae known to form symbiotic relationships with both corals and sea anemones. These nanoparticles were small enough to slip through the algae cell walls and deliver their antioxidant power inside the cell. When the team heated up the dishes, the nanoparticles kept the oxidative stress in check. When the researchers then introduced these algae to anemones lacking algae, the symbiotic partnership was restored, and the anemones lived on.
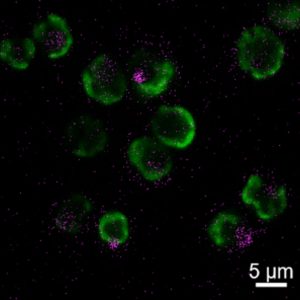
Roger’s next step is to try a similar rescue experiment with corals—a trickier procedure because corals die more quickly without their algae. She is also working to make this process more sustainable using antioxidant nanoparticles made from materials found in nature, rather than metal oxides.
Roger urges students just beginning their research careers to reach out to other scientists for help and collaboration. “I see research as just an endless suite of problems that you have to solve, and you can’t just stay in your one discipline to solve them,” she says. “Looking for collaborations is a good way of getting closer to your goal of answering the questions around these problems that we have.”
The new fuel
Chemist Gordana Dukovic, of the University of Colorado Boulder, is finding new ways to harness and store the sun’s energy. She first became interested in nanoscience around the year 2000 as an undergraduate at Rutgers University in New Jersey. At that time, nanoscience was still very much an emerging field, she says. But she was so taken with its possibilities that she went on to earn a PhD in nanoscience.

Now, Dukovic is an associate director of UC Boulder’s Renewable & Sustainable Energy Institute. She’s also a member of a nanoscience-focused Energy Frontier Research Center called Ensembles of Photosynthetic Nanoreactors. Funded by the US Department of Energy (DOE), the center’s mission is to build nanoreactors that more efficiently harness and store energy from the sun.
One of the big challenges with solar energy is how to store it. Solar panels convert the sun’s energy into electricity, which is often stored in batteries. But batteries have limited capacity for storage and transport.
Dukovic instead wants to convert energy from the sun into fuel. She starts by creating nanocrystals made from materials such as cadmium sulfide. These nanocrystals absorb sunlight, which excites the nanocrystals’ electrons. The energy from these excited electrons then drives chemical reactions between other molecules in the mix. “In that way, we store the energy from light inside the chemical bonds that are created,” explains Dukovic. The resulting product is a solar fuel, which can be transported to wherever energy is needed.
You won’t find this technology in use just yet. “We are at a basic energy research stage, where we are learning how to control materials and combine them with other kinds of materials to increase efficiencies of these desired photochemical pathways,” she says.
There are many opportunities to get involved in climate-focused nanoscience research, says Dukovic. Universities, companies, and government labs are all exploring this technology. Undergraduates interested in applying for climate-focused nanoscience jobs or graduate programs can check out journals like ACS Nano and Nano Letters to read the latest findings and see whose research excites them most, says Dukovic.

Catalyzing change
Chemical engineer Noah Malmstadt, of the University of Southern California, came to nanoscience by way of collaboration. He had long been fascinated by chemical engineering.
Around 2008, as a young faculty member at USC, he was working to create new techniques for manufacturing chemicals using microfluidics—a technique that involves processing tiny volumes of fluids in super-narrow channels. He found a colleague, USC chemist Richard Brutchey, who knew nanomaterial chemistry very well. “So, it just fit together,” says Malmstadt.
Now, Malmstadt uses nanotechnology to turn emissions into fuel. The research, funded in part by the DOE’s National Renewable Energy Laboratory, hinges on designing new nanoparticles that work as catalysts for chemical reactions.
Catalysts work by lowering the activation energy of the reaction they catalyze; they are able to participate without being consumed. “They are necessary to make modern chemistry happen because most industrial chemical reactions, in the absence of catalysts, just proceed too slowly to be practically useful,” says Malmstadt.
In one project, Malmstadt built molybdenum carbide nanoparticles that could capture carbon dioxide emissions from factories by helping to efficiently convert the gas into a hydrocarbon fuel. The nanoparticle makes it easier for the system to pull an oxygen atom off of the carbon dioxide molecule and add a hydrogen.
The scientists are also aiming to target other industrially released gases, such as methanol, which could potentially be transformed into methane and burned as fuel. “You are basically cycling the carbon,” says Malmstadt. “There are always going to be some processes that need to burn a fuel and produce carbon. We can’t move away from that entirely. But if we can capture the carbon that is being emitted by those processes and convert it, then you’ve diverted the carbon from the atmosphere.”
There are many ways to address the climate crisis, says Malmstadt. He urges students not to overlook chemical manufacturing as one possible career focus. “Anything that increases the efficiency of a manufacturing process is going to reduce energy usage,” he says.
Shrinking energy use
Nanoscientist and materials scientist Yury Gogotsi, a professor at Drexel University in Pennsylvania, is developing a class of nanomaterials that he calls MXenes, which could shrink the size of electronics and decrease the amount of energy required to produce devices. “The climate crisis we have now is because we consume and waste lots of energy. Energy is critically needed for industrial growth, but the more materials you produce, the more energy you need,” says Gogotsi.
MXenes are sheets—only three to nine atomic layers thick—made from transition metals and carbon or nitrogen, like titanium carbide. Gogotsi forms these sheets into films, coatings, wires, and textiles that conduct electricity. “Every battery in your cell phone and your computer has lots of metal foils as a current collector,” says Gogotsi. Replacing these foils with MXenes, which are 10 times thinner, could shrink the battery size by at least 10 percent and also decrease the weight, he says.
Gogotsi first became interested in nanoscience after making a serendipitous discovery. As a postdoctoral fellow at the Tokyo Institute of Technology, he studied the corrosion of silicon carbide structural ceramics and composites in water cooling zones in nuclear power plants. Previously, researchers believed that these ceramics corroded into silica fragments when exposed to supercritical water—dense water vapor at temperatures far beyond the boiling point. Instead, Gogotsi discovered that silicon from the ceramics simply dissolves. What is produced instead is a carbon structure with nanoscale pores.
Gogotsi found that he could use this previously undiscovered nanoporous carbon as a tool for storing electrochemical energy. “This research started 30 years ago, and I have been working on nanomaterials ever since,” he says.
Students who pursue careers in manufacturing will find that nanoscience is a major part of revolutionizing many industrial processes, says Gogotsi. “We are excited about the opportunities to really make major changes in the world by introducing new nanomaterials.”