Periodic Table Scavenger Hunt

Celebrate the International Year of the Periodic Table of Chemical Elements (#IYPT2019) at a chapter event, meeting, or recruiting event with this fun scavenger hunt!
Objective
How well do you know the elements around you? Identify elements that make up your surroundings in a set amount of time.
How to play
Use the periodic table scorecard to mark off the elements that you find around you. The team or person with the largest number of identifiable elements wins.
Prizes
Visit the ACS store to find prizes.
Where to Find Elements
In the human body
About 25 elements are found in the human body. You can probably already guess the main components: carbon, hydrogen, oxygen, and nitrogen account for about 96% of your body mass. You’ll come up with more elements if you consider the composition of teeth, bones, blood, and DNA.
But did you take cobalt into account? About 1 mg of cobalt is present in your body as vitamin B12. It acts as a co-enzyme to help your body make red blood cells and DNA. Although there are cobalt minerals, the only sources of cobalt that humans can digest are in meat, eggs, and dairy products (which is why it’s so important for vegans to take vitamin B12 supplements).
Your body also relies on tiny amounts of sodium, potassium, chlorine, and calcium ions to regulate nerve activity. Neurons transmit electrical signals by controlling the relative concentrations of these ions inside and outside of the cell. The right concentrations produce a voltage difference in neuron cell membranes that transmits an electrical signal from one neuron to the next.
Can you identify the rest of the elements in your body?
In the air
No doubt, you are aware that oxygen and nitrogen comprise about 99% of the air we breathe. But there are a lot of other elements that account for that last 1%. Can you figure out what they are?
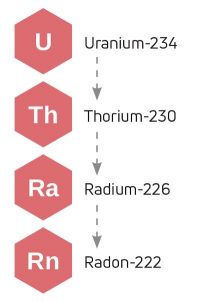
If you are in a room near the ground, you may need to consider radon. It is a noble gas. And yes, it’s radioactive. Naturally occurring 234U decays into 230Th, which further decays into 226Ra, which produces 222Rn. This decay series takes place in the soil and in certain rocks, such as granite, gneiss, and limestone, which is how it may get into your air.
The half-life of this isotope of radon is just less than four days, so in an open area, it disperses quickly. However, radon is one of the densest gases in the atmosphere, so it can become concentrated in environments like mines, underground springs, and even basements. The U.S. Environmental Protection Agency recommends that all homes be tested for radon.
In your pockets
If someone has a smartphone, you can account for up to 70 elements. You’ve probably already guessed silicon in the circuits, and oxygen (bonded to the silicon) in the glass screen. The light displays rely on the same light-emitting diode (LED) chemistry as modern lights, so you can find a lot of the same elements there (see the next page).
But did you look for the neodymium, terbium, and dysprosium in the phone’s vibrating unit? The vibration is produced by a very tiny, unbalanced motor. The motor spins when activated by the flux generated by a disk magnet. Rare earth magnets, such as Nd2Fe14B, are commonly used for this purpose because they are permanent and have a strong magnetic field. Neodymium and iron each have four unpaired electrons, so they are paramagnetic (attracted to magnetic fields) to begin with. In addition, rare earth metals are highly responsive to magnetic fields; terbium visibly expands and contracts in their presence. When these elements are combined in an alloy, they take on a crystalline structure that directs the magnetism of the individual components along a specific axis, making the material ferromagnetic (permanently magnetic), with a very strong magnetic field.
Don’t forget any credit cards or key cards. Their paints and magnetic strips contain elements. The same goes for keys and any gems.
In a classroom
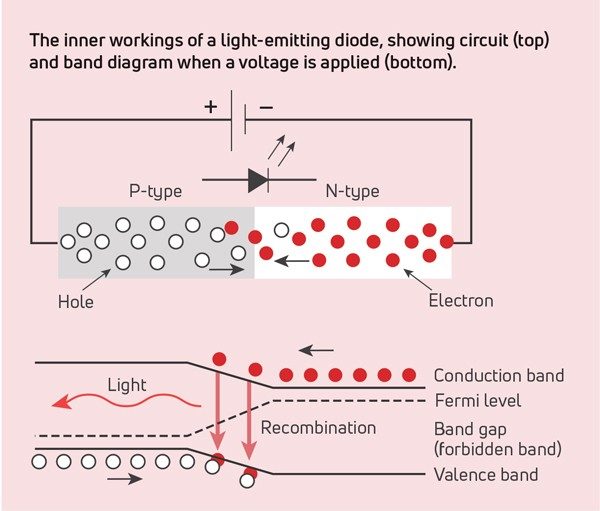
Did you check the lighting? Incandescent light bulbs use tungsten filaments, and fluorescent lighting excites mercury vapor to produce light. The newest lights rely on light-emitting diodes (LEDs). The light from LEDs is a result of the electronic band structure (the quantized energy of the electrons) of their composite materials. Incoming energy is absorbed by an electron in the valence band, which then jumps to the conduction band. This leaves a positively charged hole in the valence band. The hole and electron travel through the crystal lattice together. However, an impurity in the lattice disrupts the travel. The electron recombines with the hole and releases some energy. If the energy released is in the visible range, the material can be used in LEDs.
LEDs are made of a variety of elements, depending on the desired color. For example, red light is a result of europium doping. When small amounts of Eu2O3 are doped into yttrium oxides, the large europium atoms provide disruptions in the lattice of the smaller yttrium atoms. The resulting wavelength is in the red region of visible light when activated by electrical energy. Divalent europium can produce a variety of colors, depending on the material is it doped into. The wavelength of the energy released depends on the composition of the crystal lattice. To vary the resulting wavelength, a lattice can be tuned by using different dopants. Components that emit different wavelengths can be combined to produce a desired light effect.
What other elements can you find?
Once you start looking, you’ll find a wide array of elements almost anywhere!
References
1. Elements in the Human Body. https://askabiologist.asu.edu/content/atoms-life
2. Composition of Dry Air. https://eesc.columbia.edu/courses/ees/slides/climate/table_1.html
3. The Chemical Elements of a Smartphone. https://www.compoundchem.com/2014/02/19/the-chemical-elements-of-a-smartphone/
4. The Chemistry of Gemstone Colours. https://i1.wp.com/www.compoundchem.com/wp-content/uploads/2014/06/The-Chemistry-of-Gemstone-Colours-2016.png
5. Periodic Table with pictures of locations of where to find the elements. https://elements.wlonk.com/
6. The Disappearing Spoon and Other True Tales of Madness, Love, and the History of the World from the Periodic Table of the Elements by Sam Kean. Back Bay Books; 2011.
Learn how the American Chemical Society and others will be celebrating the International Year of the Periodic Table at www.acs.org/iypt.