2021 Heroes of Chemistry Make Huge Advances in Medicine
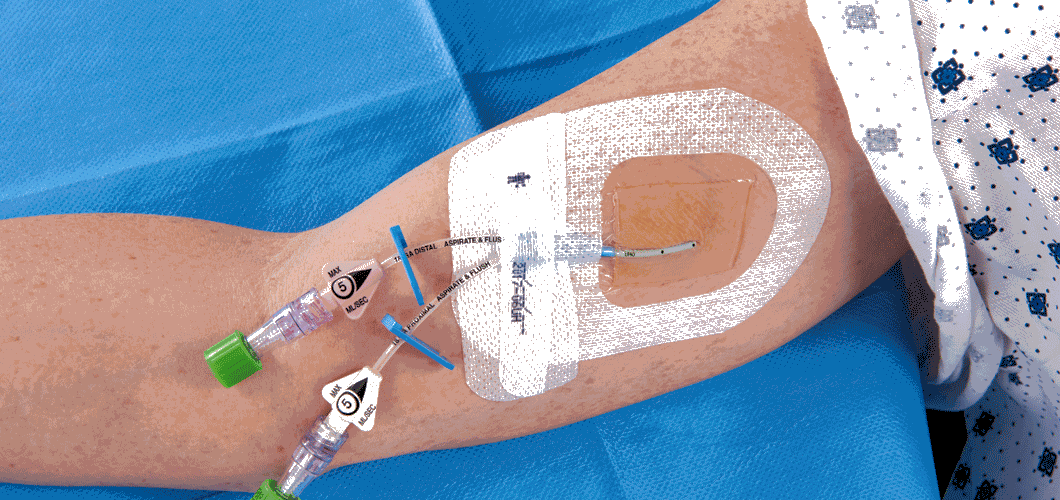
Chemists can be heroes, especially in pharmaceutical labs. They do all the heavy lifting developing ways to convert simple molecules into lifesaving medicines. The intricate and challenging work behind such astounding advancements as designing vaccines and treatments for COVID at near-breakneck speed demonstrates chemists’ extreme devotion to human life and safety.
Every drug that clears regulatory hurdles has journeyed immense distances from a chemist’s mind to a patient’s body. Even the newest approved pharmaceuticals, including the COVID vaccines, have been in the works for years—or decades.
“It's amazing,” says Andy Souers, a Distinguished Research Fellow at AbbVie who helped create the cancer treatment venetoclax. “Chemistry has a chance to make something that can literally change the course of disease for some of these patients.”
The venetoclax team is one of six that were named Heroes of Chemistry in 2021. Some of these heroes help defeat cancers or heart disease. Some have invented new molecules that halt menacing diseases in their tracks. Others combine pharmaceutical weaponry into unique forms of rescue.
inChemistry spoke to the “superchemists” to find out what makes each of their solutions so special.
Restoring the balance of good and evil
Typically, any hero’s mission is to preserve life, not end it. But sometimes, for instance when dealing with a horde of immortal villians, the quest for good means tipping a balance toward death.
To chemists at AbbVie, blood cancers like B-cell lymphoma are just like zombies or vampires—they refuse to die. Normally, pro-survival proteins balance with “pro-death” proteins that help cells self-destruct. AbbVie’s scientists developed a treatment called venetoclax that restores this balance.
Consider when this equilibrium works well: when you get a sunburn, damaged skin cells die, then flake off.
In B-cell lymphoma, the immune system’s B cells have an overabundance of pro-survival Bcl-2 and Bcl-xL proteins, which relay messages within and between cells by binding to other biomolecules. They can continue amassing mutations, becoming even harsher villains. “The pro-death guys kind of beat out the pro-survival folks,” Souers says. His team carried out their heroic ploy by stomping out important cancer cell communication.
AbbVie planned to interrupt cellular conversations with a molecule that would plug a sort pocket that pro-survival proteins use to bind. “We could all of a sudden transfer the power, so to speak, back to the pro-death factors,” Souers says.
The team designed a molecule to target Bcl-2 and Bcl-xL, but then struck a problem. Although stymying Bcl communication indeed killed cancer cells, it also reduced levels of platelets, which are important for healthy clotting. They had shifted the balance too far.
As it turned out, while Bcl-xL and Bcl-2 are nearly identical in chemical structure, Bcl-2 is markedly more important to weed out. AbbVie switched their focus to Bcl-2 alone. The chemists and structural biologists needed to discover the science of why Bcl-2 binding and Bcl-xL binding differ.
“We really struggled for a very long time,” Souers says, adding that some believed Bcl-2 would be undruggable. “We were trying to do something that nature hasn't figured out how to do.”
Many of their candidate molecules failed. But then, they discovered the key to Bcl-2’s reactivity: a simple hydrogen bond with an aspartic acid in the protein’s structure. Bcl-xL doesn’t have this aspartic acid; it instead has a glutamic acid, which contains one extra carbon atom. This tiny change made all the difference between the two targets.
“You saw jaws drop,” Souers says.
AbbVie’s “hero” chemists harnessed this particular interaction with a new molecule they could manufacture and keep stable: venetoclax.
Venetoclax earned approval to treat chronic lymphocytic leukemia and acute myeloid leukemia. When combined with a cancer-fighting antibody called rituximab, it helps patients avoid chemotherapy.
Souers gets emotional thinking about trials on the first three patients with chronic lymphocytic leukemia. “They had failed multiple treatments,” he says. “The clinicians were telling us these folks were not in good shape at all.” Eight hours after one dose of the drug, more than 95% of their malignant B cells were gone.
Freeing natural defenders
Some heroes help not by defeating a villain themselves but by supporting other, more powerful characters. The immune system is a potent protector, but sometimes a disease will keep our natural defenses subdued. Much like the Avengers creating Vision to defeat Ultron in the 2015 superhero film “Avengers: Age of Ultron,” the team of scientists at Merck designed a drug called Keytruda that empowers the immune system to fight cancers itself.
“T cells in our body are basically the fighters—the immune response responder,” says Parimal Desai, a vice president at Merck who led Keytruda’s development between 2012 and 2014. “In many cancers, the T cells are not able to function. They are inhibited by what is called the PD-1 receptor.”
PD-1 receptor proteins dot the surface of cells that conduct search-and-destroy missions targeting immune cells. (They get their name from their function of programming cell death.) Healthy cells stay out of harm’s way by flashing a secret ID badge in the form of molecular ligands. Valid ligands bind PD-1 receptors, and the cell is spared.
There is one problem with binding: some cancers wear that ID badge, too. T cells roaming around these cancers become powerless—betrayed by their own anatomy. Merck’s Desai and the rest of the team hatched an escape plan for the body’s imprisoned soldiers.
Keytruda is a monoclonal antibody—a protein grown by cells in a lab. It’s designed to block PD-1 receptors. So with PD-1 no longer functional, T cells can do their job.
Once Desai’s team found that their anti-PD-1 antibodies treated melanoma without the need for chemotherapy, Merck fast-tracked the drug.
Keytruda is now used for lung cancer, stomach cancer, lymphoma, and other diseases. “Anywhere you can see the PD-1 has blocked the T cell—[wherever that is] a primary cause of the cancer,” Desai says, “this therapy can work.”
A simple, serendipitous savior
Your kidney is your body’s most important filter. It manages levels of chemicals, such as sodium and glucose, so that you can maintain homeostasis. And with a great responsibility to the body, comes great power for kidney drugs to affect other organs. Much as Spiderman uses his superpowers to fight evil villains like the Green Goblin, scientists at AstraZeneca/Bristol Myers Squibb use a new drug to leverage that great power for good.
Their drug, called Farxiga, helps the kidneys eject more glucose, sodium, and water into urine. It works by inhibiting a protein channel that would otherwise reabsorb sodium and glucose back into the blood.
Since more glucose exits the body, Farxiga helps diabetics with their blood sugar levels. While analyzing early clinical results, the companies noticed Farxiga helped with even more.
“We saw some trends to amazing results on other things—on heart failure and kidney disease—which prompted us to go all in,” says Elisabeth Björk, an MD/PhD in endocrinology and a senior vice president at AstraZeneca. Farxiga is now approved to prevent heart issues with chronic kidney disease.
Björk is proud of her team for getting to this point and is grateful that it allows her to help patients even though she is no longer a practicing physician. “Instead of treating patients one to one,” she says, “I'm now treating millions of patients.”
Heroic combination
You are probably not alone if you have felt anxiety at the point in a superhero movie where the hero saves the day, but there's still 45 minutes left in the story. The job seems done, but you know the worst is yet to come. This scenario is very familiar to medical doctors who see patients undergoing treatment for one condition but who end up acquiring a secondary infection while in the hospital. Scientists at 3M came up with a solution: a supercharged wound dressing called Tegaderm CHG Chlorhexidine Gluconate I.V. Securement Dressing that also prevents infections.
The Tegaderm CHG dressing covers and protects wounds on skin when people need catheters to deliver medication to their bloodstream. But while the Tegaderm’s barrier solves one problem, another one lingers. A physical barrier can't stop further infections on its own. These infections are called catheter-related bloodstream infections, or CRBSIs. “In the US alone, there are more than 200,000 cases of CRBSIs causing 24,000 deaths each year,” according to Ying Zhang, a biopharmaceuticals scientist at 3M.
Zhang’s team designed a solution to the CRBSI problem by combining their wound dressing with an antimicrobial gel, which consists of chlorhexidine gluconate.
Creating a perfect gel for the film was tough. The dressing had to have the right chemistry and physical properties: It had to be clear and sticky on both skin and plastic catheters, without clogging up the skin. “The chemistry had to work such that the antiseptic gel was able to rapidly deliver a highly charged and relatively unstable antimicrobial compound,” says Zhang.
The team figured it out. For the past 15 years, 3M’s Tegaderm CHG Chlorhexidine Gluconate I.V. Securement Dressing has helped millions of patients in more than 100 countries.
Double duty to save hearts
What can you do if the same thing that keeps you alive also threatens your survival? Heroes, in this case, must do two things at once: They need to free you from this double-edged sword and keep you alive with their own super abilities. Seeing an opportunity to carry out just such a rescue mission for people with cardiovascular issues, a team of chemists at Novartis designed a drug called Entresto.
As the heart weakens with age or disease, blood vessels get more narrow. That change helps the body retain salt and water. Over time though, this process hurts the heart.
The Entresto solution involves a dual-action treatment that was approved to help people fighting hypertension and heart failure. One component of the drug blocks hormones that constrict blood vessels. The other promotes healthy circulatory activity and relaxes the blood vessels. Superchemists at Novartis invented the drug by working out intricate details of the chemistry. The result was a “co-crystal,” which is a crystalline solid composed of two molecular or ionic compounds. In this case, Entresto combines sacubitril and valsartan. Designing these pharmaceutical compounds was no easy feat.
“Once we solved the X-ray crystal structure, we discovered how beautifully built this compound was,” coinventor Thomas J. Blacklock said in a 2015 interview. The compound's structure stabilizes two molecules proven to treat hypertension and heart failure.
Of the 6 million people in the US who deal with heart failure, about 5 million may benefit from Entresto's one-two punch.
Blocking unstoppable tumors
Some cancers can spread to the brain. Some develop new mutations that make them resistant to current medicine. Fortunately, one treatment addresses both of these grave concerns for non-small cell lung cancer (NSCLC).
Lung cancer kills more than 1.5 million people worldwide annually—more than any other cancer. More than 85% are non-small cell lung cancers, thousands of which are diagnosed with mutated ALK enzymes, every year. (These mutated ALK enzymes allow the cancer to replicate quickly.)
A team of Pfizer scientists developed a drug called Lorbrena. As an ALK-inhibitor, Lorbrena sticks onto the mutated protein and stops it from communicating with the rest of the cell—stopping the cancer from multiplying.
Lorbrena is a third-generation inhibitor, which means it’s designed to address mutations untreated by first- or second- generation ALK inhibitors. At the same time, this drug can get past the blood-brain barrier—a critical step for a disease that spreads to the brain in 25 to 40% of patients.
Lorbrena now has approval for treating patients newly diagnosed with ALK-positive NSCLC. “I’m excited to celebrate the accomplishments of our amazing colleagues who were recognized as Heroes of Chemistry by the American Chemical Society for their work in developing Lorbrena,” says Chris Boshoff, chief development officer in oncology at Pfizer. “This award represents the incredible dedication of our team to this community and is a testament to our legacy in developing breakthrough medicines for people with advanced disease.”