Why Adjuvants Make Vaccines Better
Throughout history, pandemics have swept the world, killing massive numbers of people. From 1346 to 1353, The Plague, better known as Black Death, traveled from Asia to Europe, where it wiped out more than half of the population. Caused by the bacterium, Yersinia pestis, it spread to humans by fleas that were infected by rodents.
From 1918 to 1920, Spanish flu took the lives of 500 million people across the globe who were infected with the H1N1 virus. Lethality was amplified by the cramped conditions of soldiers and the poor nutrition that many people experienced during World War I.
Today, we are exposed to another dangerous organism–the SARS-CoV-2 virus‒that has infected 360 million people to date and caused more than 5.6 million deaths. But, unlike the Spanish flu, Black Death, and all the pandemics in between, we have vaccines to help keep us safe.
Although the main component of a vaccine, the antigen that drives the immune response, gets most of the public’s attention, there is another component, the adjuvant, which deserves time in the spotlight. An adjuvant is a substance that improves the effectiveness of a vaccine. Not all vaccines have adjuvants, but many common ones do, such as diptheria, tetanus, and pertussis (DTaP) and hepatitis B vaccines. In these cases, the adjuvants are so important that the Food and Drug Administration (FDA) evaluates them as part of the vaccine itself.
Read on to learn more about the power of adjuvants to protect us from infection.
Vaccine development
1 oz prevention = 1 lb cure
In the battle against viruses, vaccines are faster and easier to develop than drug treatments. An antiviral drug needs to target a specific pathway that is essential to the growth and reproduction of the virus. Unfortunately, viruses are very simple, so they don’t have many unique pathways to disrupt. Worse, they rely on human cells for their growth and reproduction. Targeting those pathways without actually harming human cells is a mighty challenge.
On the other hand, a vaccine commissions your immune system to recognize a given antigen (a protein or carbohydrate from an invading virus or bacterium) as foreign and produces antibodies to combat it. Because your immune system is already primed to attack outside forces, asking it to flag a new substance as foreign is straightforward.
Once vaccinated, an individual may remain protected against a disease for years or even a lifetime. A vaccine can even eradicate a disease if it is distributed widely enough, as in the case of smallpox.
Types of vaccines being investigated or used against COVID-19
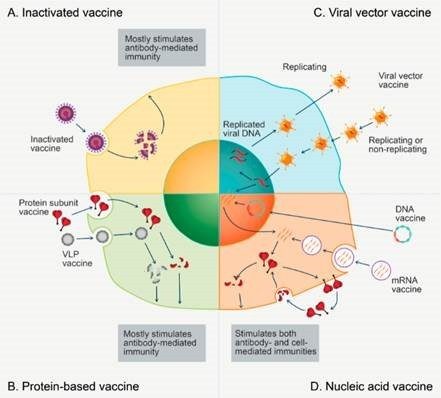
Types of vaccines being investigated or used against COVID-19
(A) An inactivated vaccine produces antigens when it is broken down by white blood cells (T cells).
(B) A protein-based vaccine produces a response focused on the molecular shape of the antigen.
(C) A viral vector vaccine delivers the DNA code of the antigen, which enhances immune response.
(D) A nucleic acid vaccine acts as the template for the synthesis of proteins as antigens.
The rise of adjuvants
Generally, an adjuvant enhances the response of the immune system to the presence of an antigen. The trick is making sure you don’t overstimulate the immune response causing it to attack and damage the body’s own tissues. Some adjuvants help stabilize the antigen for longer storage or enable it to better withstand the rigors of the body or simply help entry of the antigen into the bloodstream by injection in the liquid phase.
French veterinarian and biologist Gaston Ramon (Figure 1) is credited with the discovery of the medical value of adjuvants. In 1925, he noticed that horses vaccinated against diphtheria showed a stronger immune response when inflammation occurred at the site of injection. Ramon set out to test a range of common materials and foodstuffs for their ability to cause irritation and inflammation as vaccine additives.
On the basis of a common belief that substances safe to ingest were safe to inject, Ramon demonstrated how things like breadcrumbs, starch, agar, and soap improved antibody responses in vaccinated animals. Some modern adjuvants rely on materials that are related but are safer to inject.

A similarly serendipitous discovery followed a year later when Alexander Glenny, a British immunologist at the Wellcome laboratory in London, noticed that the aluminum salts used to purify diphtheria protein also enhanced antibody response to the vaccine. Since then, aluminum hydroxide, phosphate, and sulfate have become standard adjuvants for diphtheria, tetanus, pertussis, hepatitis, pneumococcal, and meningococcal vaccines.
Adjuvants are used mainly in vaccines in which the shape of the antigen is what triggers the immune response‒as in the distinct spike proteins that dot the exterior of the SARS-CoV-2 virus (type B vaccines shown in Figure 2). Scientists currently believe that the pattern recognition receptors (PRRs) of the innate immune response are stimulated by the adjuvant. PRRs identify the molecular patterns characteristic of individual pathogens.
Saponins in vaccines
Saponins are used as adjuvants because they are capable of triggering an intense immune response even in low doses. Saponins are a family of organic compounds known as triterpene glycosides. Saponins get their name from an ability to produce soapy foam when agitated in water. They are abundant in many plant species, dissolve in both water and fats, taste bitter, and are often toxic.
A crude saponin extract had been used in veterinary vaccines since the 1950s, but it was too toxic for humans, causing red blood cells to burst. Then, in 1991, an American research chemist research chemist, Charlotte Kensil, used chromatography to separate some of the 50 saponins present in the inner bark of Quillaja saponaria, aka Chilean soapbark trees (Figure 3). She found that one of them, QS-21, generated both an antibody and a T-cell response, with few toxic effects. GlaxoSmithKline (GSK) then licensed QS-21 (see Figure 4) and included it in a new vaccine along with a second adjuvant, a fatlike substance derived from Salmonella bacteria.
Three years ago, this potent combination was brought to the market in the shingles vaccine Shingrix. Shingrix vaccine conferred immunity in 91 percent of people over 70 years old, more than double that of a previous shingles vaccine. In 2021, Mosquirix, another GSK formulation containing the same combination of adjuvants, became the first vaccine endorsed by the World Health Organization for the prevention of malaria.

Figure 3. The leaves and flowers of Quillaja saponaria
Credit: Franz Xaver, GFDL, GNU Free Documentation License, Creative Commons
Managing the supply chain
No matter how effective a vaccine is, it will not put a dent in any disease unless it can be produced on a massive scale. Nine years ago, researchers estimated that the global supply of Quillaja extract of pharmaceutical-grade QS-21 was sufficient for just 6 million doses of vaccine. So, even if you could harvest all of the available QS-21 in the world, all you would really accomplish is destroying an entire plant species. (In the 1980s and 1990s, the Pacific yew tree was similarly threatened when it was found to be a good source of the anticancer drug paclitaxel.)
Phyton Biotech has developed cultured plant cells to produce the necessary saponins. Other companies, such as GSK and Sanofi, are exploring alternative sources by using a less potent adjuvant based on an emulsion of water and oil from shark liver.
Even more promising is a branch of chemistry called natural product research, in which chemists develop fully synthetic routes to compounds found in nature. Different (and more abundant) conformers of saponins are also being investigated. These studies have not only provided expedient access to adjuvant-active saponins but have also yielded insights into activity at the molecular level that correlates with in vivo medical properties.
Although the biological and medical effects of individual adjuvants are known, details of the mechanisms in biochemistry at the molecular level by which they influence the human immune system and relate to specific antigens are not well understood. This applies even to the aluminum-based adjuvants discovered nearly a century ago. Saponins, however, are known to be surfactants, which may shed some light on the particular mode of action of QS-21 as an adjuvant. It is possible that the soapy property of saponins in water enables molecules to surround the large subunits of protein of the antigen to allow the antigen to enter the liquid phase for injection into the bloodstream.
Much work still remains to be done to understand fully such powerful vaccine add-ins!
Know Your Triterpene Glycosides
Saponins are a broad group of molecules, but they all have two main components: a terpene (or, rather three terpenes) and a carbohydrate.
Terpenes are common in nature and are polymers of isoprene molecules, CH2C(CH3)CHCH2. The simplest terpene, known as a monoterpene, has 10 carbon atoms and consists of 2 isoprene units. The monoterpenoids, camphor and menthol, are used to clear mucus from sinuses during cold and allergy seasons.
Triterpenes, molecular formula, C30H48, consist of three terpene segments, so there are six isoprene units. The triterpene, squalene, is presented below and is made up of six repeat units of isoprene. Steroids are derivatives of linear molecules of squalene (see structural formula below) but are based upon the skeleton of a pentacyclic carbon ring. Carbon atoms making up six-ring (hexacyclic) triterpenes are particularly relevant to studies of saponins.
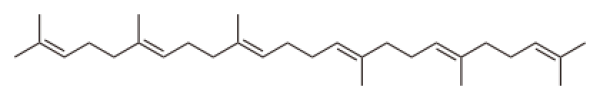
A glycoside is formed when a sugar molecule replaces a hydroxyl on another molecule to form a carbon-oxygen-carbon linkage, known as a glycoside bond. This covalent bond resembles the carbon-oxygen-carbon bond present in a molecule of ether.
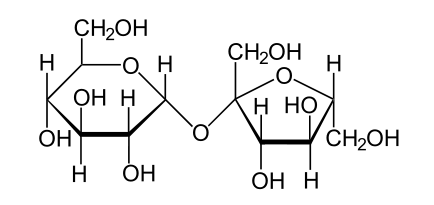
Sucrose (table sugar) is a familiar example; it comprises glucose and fructose joined by a glycoside bond.
Lactose has a glycoside bond and is the sugar found in the animal kingdom. It is present in small concentrations in blood plasma and in the milk of mammals.
Saponins are a group of triterpene glycosides found in nature. Commercial applications range from generating the foamy head on root beer to fire extinguishers. The application of saponins as vaccine adjuvants continues to be an active area of research.
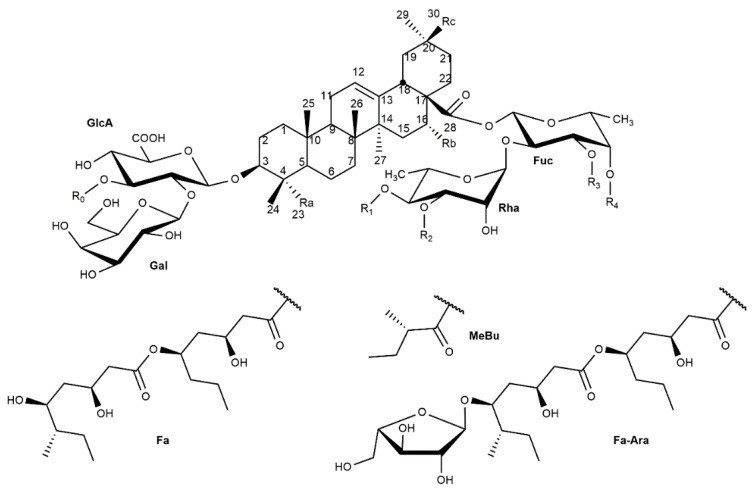
Figure 4. General structure of saponins extracted from Q. saponaria and Q. brasiliensis
References
Fleck, J.D., et al. Saponins from Quillaja saponaria and Quillaja brasiliensis: Particular Chemical Characteristics and Biological Activities. Molecules 2019, 24(1), 171.
Kensil, C.R. Separation and characterization of saponins with adjuvant activity from Quillaja saponaria. J. Immunol. 1991, 146(2), 431–437.
Zhu, D.; Tuo, W. QS-21: A Potent Vaccine Adjuvant. Nat. Prod. Chem. Res. 2016, 3(4), 113.
Pulendran, B.; Arunachalam, P.S.; Higgins, D.T. Emerging Concepts in the Science of Vaccine Adjuvants. Nat. Rev. Drug. Discov. 2021, 20, 454-475.
Liao, Y., et al. Saponin surfactants used in drug delivery systems: A new application for natural medicine components. Int. J. Pharm. 2021, 603, 120709. DOI: 10.1016/j.ijpharm.2021.120709
Ghirardello, M., et al. Exploiting structure–activity relationships of QS-21 in the design and synthesis of streamlined saponin vaccine adjuvants. Chem. Commun. 2020, 56, 719-722. DOI: 10.1039/C9CC07781B