Dear Sunscreen: Bring the Shade!
Come summertime, everyone loves getting out for fun in the sun. The time we spend outdoors has great health benefits — vitamin D production, improved creativity, stress relief. But outdoor activity also exposes our skin and eyes to harmful ultraviolet (UV) light from the sun and without proper protection, we risk skin damage, cataracts, and even cancer.
Danger in the sun
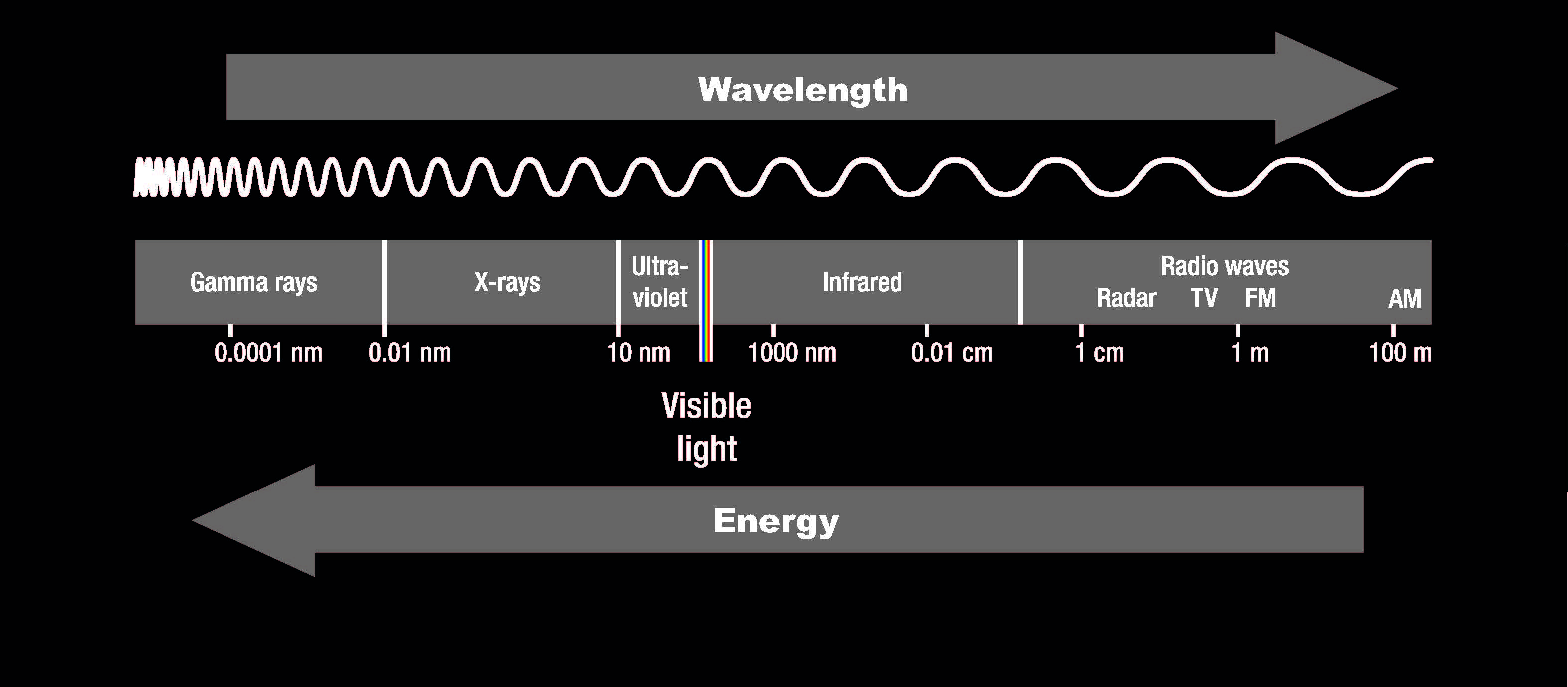
The sun’s light includes energy from the entire electromagnetic spectrum; however, UV radiation is the real danger. Ultraviolet radiation is higher in energy than visible light. The energy of light is equal to Planck’s constant multiplied by frequency. Since the speed of light equals frequency multiplied by wavelength, energy is inversely proportional to the wavelength. In other words, shorter wavelengths mean higher energy:
c = λν and E = hν so E = h(c/λ)
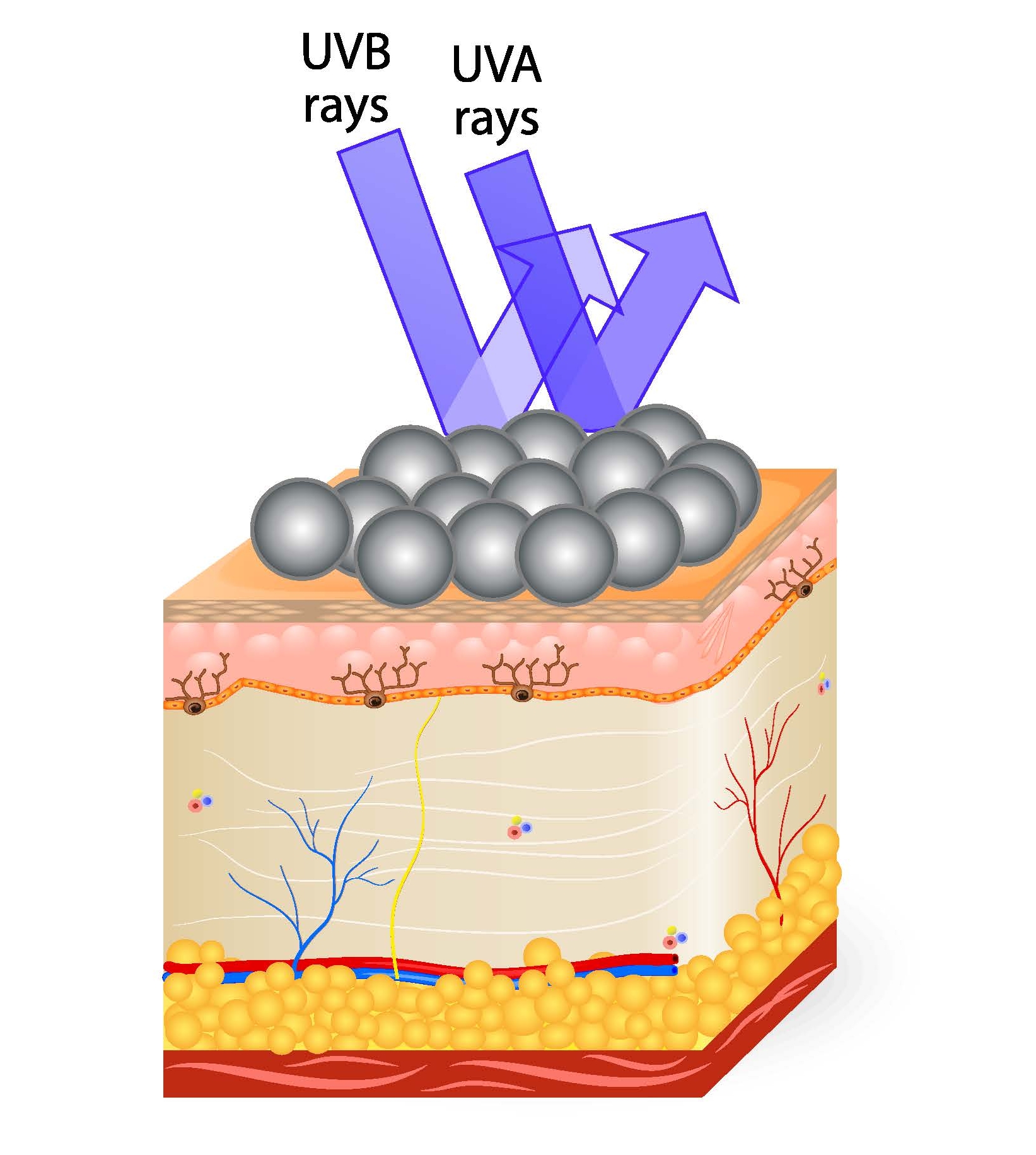
The wavelengths of UV coming from the sun are classified as UV-A (320–400 nm), UV-B (290–320 nm) and UV-C (100–290 nm). UV-C has the highest energy, making it the most dangerous of the three wavelength types. Luckily, the earth’s ozone layer provides protection from UV-C (light that is even higher energy than UV is absorbed by nitrogen in the atmosphere), leaving UV-A and UV-B wavelengths to be of concern. The high energy of photons of UV light can penetrate the skin and trigger damaging reactions reactions within skin’s DNA or excite other chromophores present in the skin to form reactive oxygen species (ROS). ROS can go on to produce other damaging reactions in the skin. UV-A penetrates the skin more deeply than UV-B. How deep the damage to the skin is dependent on the energy of the photon.
| UV category | Wavelength range | Effects | Protection |
|---|---|---|---|
| UV-A | 320–400 nm | Tanning Premature skin aging Skin cancer | Avobenzone (Parsol 1789) Ecamsul (Mexoryl) Zinc oxide |
| UV-B | 290–320 nm | Vitamin D production Sunburn Cataracts Genetic damage Skin cancer | Oxybenzone Homosalate Octinoxate Octisalate Titanium dioxide |
| UV-C | 100–290 nm | Cellular decomposition | Ozone in lower stratosphere |
Ninety-five percent of UV radiation that reaches the earth’s surface is UV-A radiation. UV-A radiation triggers the tanning reactions in skin, as the skin darkens in an attempt to protect itself from further damage. UV-A plays a major role in skin aging. It also causes damage to the epidermis (the outermost layer of the skin) and has been shown to be a factor in the development of skin cancers.
UV-B radiation is responsible for reddening or burning of the skin and also plays a key role in the development of skin cancers.
The chemistry of protection
So what does the skin really need for protection? The answer is shade. You could try staying inside — away from UV radiation — but you lose the benefits of the outdoors; plus, standard glass windows only absorb UV-B radiation. Covering up while you’re outside can also help, but the protection provided by clothing varies greatly. Chemists have developed sunscreens to fill in the gaps in your UV protection.
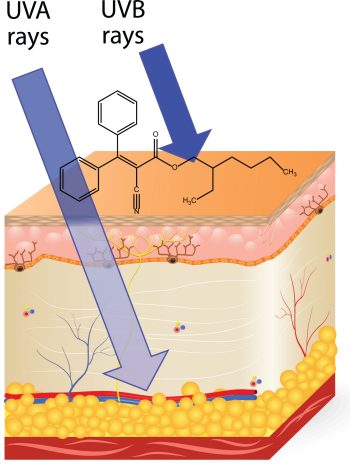
There are 17 active ingredients approved by the Food and Drug Administration for use in sunscreens. The two approved inorganic ingredients, titanium dioxide and zinc oxide, work by reflecting and scattering the radiation before it reaches the skin. Of the remaining active ingredients, the most commonly used are avobenzone, dioxybenzone, Ecamsule (Mexoryl SX), oxybenzone, octinoxate, octisalate, homosalate, and octocrylene. These molecules absorb UV photons, enter into an excited state, and then dissipate that energy by an alternate pathway.
Different molecules absorb photons of different wavelengths. Avobenzone captures photons in the 340 to 400 nm range. Ecamsul (Mexoryl SX) is a broader-spectrum filter and can capture photons in the 290 to 340 nm range. Dioxybenzone and oxybenzone capture photons in the 290 to 320 nm range. Commercial formulations may include several active ingredients to achieve the desired efficacy.
Putting the chemistry to work for you
A sunscreen product’s efficacy is commonly indicated by its “sun protection factor” or SPF. The SPF is an indication of how long it will take for UV-B rays to begin to redden the skin. Skin coated with an SPF 15 sunscreen will take 15 times longer to redden than without the sunscreen. So, if you normally start to redden after 10 minutes in the sun, an SPF 30 sunscreen should protect you for 30 x 10 minutes, or 5 hours … right? Not quite.
Even water-resistant sunscreens get rubbed off by sweat, washing, entering the water, or mopping your skin. So don’t let a high SPF fool you into a false sense of security. Apply generous amounts of sunscreen at least 20 minutes before sun exposure to give the ingredients time to bind to your skin, and then re-apply every two hours. In fact, you should re-apply the sunscreen even more frequently if you are swimming, sweating, rubbing your skin, or using additional protection (such as insect repellent) that can dilute the sunscreen. Also, look for sunscreens with a variety of ingredients to ensure broad-spectrum protection. Because organic compounds bind better to skin than inorganic ones, make sure your sunscreen contains at least one organic radiation filter.
The darker side of sunscreen?
Sunscreens aren’t perfect. For example, zinc oxide and titanium dioxide scatter both UV and visible light; they are even used in many household products to provide a whiter, brighter appearance (check the ingredient list for your toothpaste, headache medicine, Kool-Aid, etc.). To prevent sunscreens from looking opaque, manufacturers turn to nanoparticles smaller than the wavelength of visible light. But these smaller particles can accumulate in hair follicles and penetrate the skin. The impacts of such effects are still under investigation.
UV radiation of the organic sunscreen ingredients can result in the breakdown of the molecules into byproducts or ROS (free radicals) that can, in turn, produce adverse effects. There is also the potential for the compounds to be absorbed into the skin and transported to other tissues, urine, and breast milk.
Certain sunscreen ingredients have undesirable environmental effects. For example, because of observed damage to coral reefs, the State of Hawaii has been recommending that individuals who swim in the waters around Hawaii not use sunscreens that contain oxybenzone as an active ingredient.
Sunscreens of the future
Improving sunscreens is an active area of research. Vasillios Stavros at the University of Warwick (U.K.) studies the mechanisms by which filters dissipate the energy from the absorbed UV light. By determining which compounds are likely to break down or remain in an excited state, Stavros hopes to identify more stable sunscreen compounds. Others are looking at synergistic effects of other ingredients like lignin. New research by Shiping Zhu, et.al., has shown that adding one percent organosolv lignin can increase the SPF, while another lignin, lignosulfonate, causes the same product to separate.
Mark Saltzman at Yale is investigating the use of transparent bioadhesive nanoparticles as a way to prevent active ingredients in sunscreens from entering the skin. The bioadhesives adhere to the skin, keeping organic filter molecules in place. This approach has the added benefits of requiring a smaller amount of active ingredients in sunscreen products, and also reducing the user’s exposure to them.
Nature may have answers, too. Cyanobacteria and algae produce are natural sunscreen molecules. A team led by Diego Sampedro at the University of La Rioja is looking at mycosporine-like amino acids as potential substitutes for the current compounds.
The Centers for Disease Control (CDC), National Institute for Occupational Safety and Health (NIOSH), and the Skin Cancer Foundation recommend that you avoid prolonged exposure to the sun when possible. But when you do go out, use proper clothing, sunglasses, and sunscreen to help you safely take advantage of summer’s sweet, sweet sunshine.