How To Survive Your Organic Chemistry Class
If you’ve got an organic chemistry course coming up, chances are you’re either excited for a second year of chemistry or dreading the whole thing. Often called “orgo” or “o-chem,” this two-semester introduction to the synthesis, reactions, and analysis of carbon-based molecules has acquired a negative reputation.
Orgo can be particularly daunting to students who don't have an innate passion for the topic. It is a common requirement for many programs, such as premed (as a foundation for the biochemistry that runs the body), biology and environmental science majors (biology is mostly macroscale organic chemistry), forensics majors (human biochemistry again), and other science disciplines. Even chemistry majors might not click with the o-chem curriculum right away like they do with other chem classes, especially students who are more interested in the rest of the periodic table than in studying carbon so closely.
Still, with our tips, it is possible to make friends with organic chemistry, which touches so many parts of our everyday lives.
Let’s get started!
Learn the vocabulary
Organic chemistry has a reputation for requiring a lot of memorization, and it certainly can seem that way in the beginning. But what you are actually learning is basic vocabulary and a few related scientific concepts. Once you learn terms and concepts, you can combine them in myriad ways to describe the contents of and reactions in your Erlenmeyer flask.
In orgo, there are three buckets of vocabulary you’ll need to know:
∎ Nomenclature
By definition, organic molecules are carbon-based, but the carbon atoms are often bonded to a few other atoms like hydrogen, oxygen, nitrogen, and sulfur. Functional groups (common arrangements of atoms, like a chain of six carbons, a circle of carbons, or an added -OH group) are given their own prefix or suffix. Learn the prefixes and suffixes, and you will be able to put them together to form the complex compound names you see throughout the course.
∎ Reactions
Although a typical organic chemistry course covers 200-300 reactions, you actually only need to learn a few types (see next section). Once you have learned the different reaction types, you’ll be able to figure out what is happening in most reactions.
∎ Analysis
Most organic chemistry courses cover a few analytical techniques frequently used throughout the science, namely, mass spectrometry, infrared spectroscopy, and nuclear magnetic resonance. For each of these techniques, you’ll need to recognize common features and patterns that indicate different types of molecular structures. As with nomenclature, learning basic analytical techniques will help you determine a molecule from a few key pieces of information.
This may seem like a lot to absorb, but unless you are taking a compressed version of orgo, you will learn all of this vocabulary over the course of an academic year. If you find yourself struggling, get help immediately! Go to a tutor, your professor’s office hours, or a knowledgeable classmate. Like calculus, organic chemistry builds on itself, and you’ll fall behind quickly if you don’t keep up.
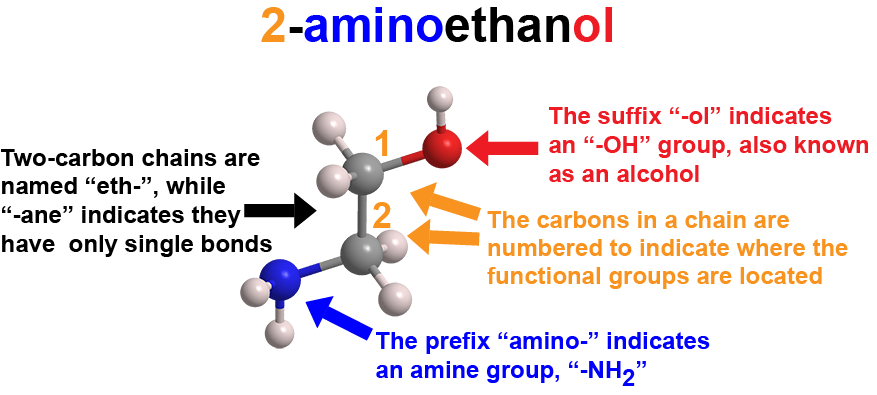
In organic chemistry, a compound’s name tells you its structure and composition. This is how to translate the name of 2-aminoethanol, aka ethanolamine (C2H7NO), a viscous, basic liquid currently used in carbon capture technology and as a possible indicator of protein precursors in outer space.
Learn to recognize the patterns
When you learn a language, you not only learn vocabulary, you also learn the system of how to put words together to communicate. This is how “querer,” “tomar,” and “taza” become “¿Quieres ir a tomar una taza de cafe?” (“Want to go grab a cup of coffee?”).
Similarly, the differences in electronegativity also dictate your analytical results. Once you learn how the electrons move, you will also be able to predict how those differences will affect your analysis.
There are a small number of fundamental mechanisms (i.e., ways that bonds change) in organic reactions--most important, proton transfer, substitution, elimination, and addition. These processes get combined into more complex reactions, much like the notes of a scale get combined into a melody. Start by learning the fundamentals.
Here’s an example: The breaking and formation of bonds is simply the electrons in the bonds moving from a less electronegative atom to a more electronegative atom. So, once you learn how electrons move and the related thermodynamics (energy transfer) in the movement, you will be able to predict the outcome of a number of reactions.
Similarly, the differences in electronegativity also dictate your analytical results. Once you learn how the electrons move, you will also be able to predict how those differences will affect your analysis.
Yes, the first couple of weeks in orgo will have you learning a whole new vocabulary. The best thing you can do is start looking for patterns right away. As you learn the patterns, you will find that there will be less need for memorization. Again, if the patterns aren’t making sense to you, get help! It will save you a lot of work later on.
Patterns in action
The following diagram represents six of the reactions you will learn in organic chemistry.
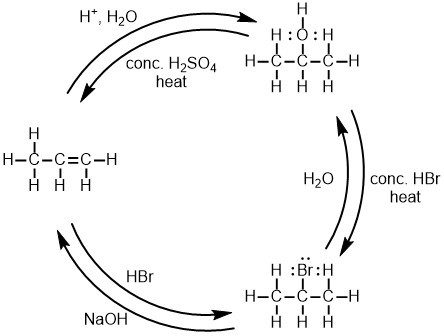
Although it may be tempting to simply memorize this diagram and move on, you’ll have a much easier time if you stop to notice a few patterns.
First, though there are six reactions depicted in the diagram above, there are only three types of reactions. Going from 1-propene (the left-hand compound) to either 2-propanol (the upper-right compound) or 2-bromopropane (the lower-right compound) involves addition, starting with a carbon-carbon double bond and adding atoms.
Going from either 2-propanol or 2-bromopropane to 1-propene involves elimination — removing atoms to make a carbon-carbon double bond. Addition and elimination reactions are opposite processes.
Finally, going from 2-propanol to 2-bromopropane, or from 2-bromopropane to 2-propanol, involves substitution — replacing some atoms with other atoms. These three processes (aka “mechanisms”) form the basis for many organic reactions.
Now let’s look specifically at the reaction going from 1-propene to 2-propanol via reaction with acid (the H+):
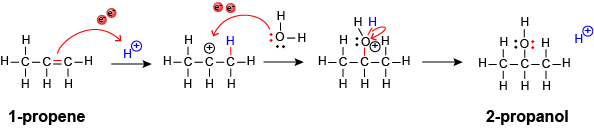
Remember from General Chemistry that H+ is positively charged and therefore a good Lewis acid (or electron acceptor). Meanwhile, the double bond in the 1-propene includes four electrons shared between two atoms, which is an area of high “electron density.” High electron density generally makes for a good Lewis base (or electron donor). The Lewis base (the double bond of 1-propene) reaches out to the Lewis acid (the H+) to make a new carbon-hydrogen bond. But after this happens, the 1-propene has “lost” electron density, so it becomes a good Lewis acid (electron-pair acceptor). In the second step, a water molecule can act as a Lewis base, where one of the oxygen lone pairs reaches out to become an oxygen-carbon bond (shared pair of electrons). A final step breaks an oxygen-hydrogen bond to get back to having two bonds and two lone pairs on the oxygen. This is the standard bonding pattern for oxygen (just like four bonds for carbon or one bond for hydrogen).
This mechanism isn’t only true for going from 1-propene to 2-propanol. The same steps happen in the reaction to go from 1-propene to 2-bromopropane, as well as many other addition reactions. Additionally, while the mechanism of the 2-bromopropane-to-2-propanol-in-water reaction is slightly different, if relies on the same Lewis acid/base considerations discussed above. So, by learning one mechanism, you actually learn several reactions!
Now, see if you can deduce the mechanisms for the other three reactions in the diagram. And, if you can’t now, be sure you can by the end of the semester.
Get active and creative
Passive learning—rereading your text or notes—is generally considered the absolute worst way to learn something, including orgo. For effective learning (and the fastest way to that A on your exam), active learning is how you will succeed.
Here are some great active studying techniques for orgo:
∎ Make something
Create your own infographic of functional groups or spectral shifts. Develop a study sheet for your classmates. Create your own digital flash cards. Draw your own chart of how the reaction mechanisms connect the different reactions you learn.
∎ Get visual
Build molecular models for compounds and reactions you come across. Your textbook, notes, and professor’s presentation may be two-dimensional, but chemistry happens in three dimensions and can be understood best by visualizing three dimensions. Building models will help you visualize what’s happening so you can understand it better.
∎ Teach it
Start or join a study group and practice explaining orgo to your classmates. If you have a very patient friend, you can teach them. Or try putting your teachings on a TikTok or blog post to teach complete strangers. The process of explaining the information will help keep it clear in your head.
∎ Practice, practice, practice
The more practice problems you do, the better. Use your textbook, old exams, and library and online resources. Give yourself a time limit to finish the questions. Wait until you are completely done to check your answers and go back over anything you missed. This will help ensure that you can do questions in the allotted timeframe you would be given in an exam while retaining the concepts you are learning.
Be sure to seek out “synthesis” problems. In these problems, you are asked to describe how you could synthesize a certain product using one or two reactants. Frequently, these problems draw on reactions you learned in previous chapters, and the only way to get good at combining all of this knowledge is to practice it. If you can, look for problems that allow you to practice and get comfortable with reaction mechanisms (e.g., addition reactions, elimination reactions, substitution reactions, pericyclic reactions).
Remember your general chemistry
There’s a reason general chemistry is considered an introductory course. General chemistry introduced you to a lot of concepts you are going to continue using in organic, including:
Thermodynamics
Orgo is about reactions. Understanding the interplay of energy, enthalpy, and entropy that drives them will help you learn the reactions more easily.
Electronegativity and electron transfer
Chemical reactions are really just the movements of electrons among atoms and molecules. Electronegativity and electron transfer help you predict where electrons will move next.
VSEPR theory, Lewis diagrams, isomers
Carbon is unique among the elements in its ability to form an immense variety of chains, shapes, and configurations, each of which has unique reactivity. Being able to understand, predict, and draw these shapes will help you understand reactions better.
Unit conversions, stoichiometry, dilutions
These concepts will remain useful for classwork, but you will use them more in your lab work, which becomes more independent in orgo.
So, hang on to your general chemistry text and notes—or at least find a text in the library—to brush up on previously learned material as needed.
You may also need to look back at any literature or writing-focused classes you’ve taken. For many students, organic chemistry marks the introduction to formal lab reports. These are short papers written in the style of a scientific paper.
Breathe!
Seriously. Every day, step away from your study area and take a real break. Take a walk, go to a campus spin class, do some yoga—anything to get the blood flowing. Paint a picture, go dancing, hang out with friends. Eat healthy and get plenty of sleep. Manage your stress with friends, family, or even therapy. Do whatever you need to do so that your brain and spirit are nourished along with your body.
Taking care of yourself will help you learn at peak effectiveness and be happier while you do it.
With a little help, and a lot of patience, you can rock the organic chemistry curriculum!